Table 1.
Dependence on Solvent and Liganda
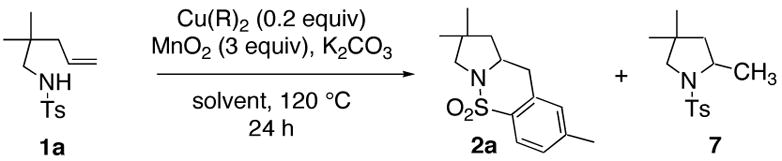 | |||||
|---|---|---|---|---|---|
| entry | R | solvent | ligand (equiv) | yield (%) 2 | yield (%) 7 |
| 1 | EH | PhCH3 | -- | <5 | trace |
| 2 | EH | PhCF3 | -- | 7 | trace |
| 3 | EH | PhCH3 | 8 (0.2) | 41 | 7 |
| 4 | EH | PhCH3 | 8 (0.8) | 63 | 17 |
| 5 | EH | PhCF3 | 8 (0.8) | 75 | <5 |
| 6 | OTf | PhCF3 | 8 (0.8) | 29 | <5 |
| 7 | OTf | PhCH3 | 9 (0.2) | 59 | 12 |
| 8 | OTf | PhCF3 | 9 (0.2) | 60 | <5 |
Reaction conditions: Substrate 1a was dissolved in solvent (0.1 M) and treated with K2CO3 (1 equiv), MnO2 (3 equiv), CuR2 (0.2 equiv) and the specified amount of ligand and stirred in a sealed tube at 120 °C for 24 h.
Yields refer to amount of compound isolated after chromatography on SiO2. Remainder of material was always unreacted starting 1a. EH = 2-ethylhexanoate.
