Table 3.
Scope of Enantioselective Carboamination with (R,R)-11aa
| entry | substrate | product | yield (%)b | ee (%)c | ERc |
|---|---|---|---|---|---|
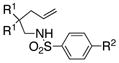
|
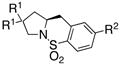
|
||||
| 1 | 1a, R1 = Me, R2 = Me | 2a | 85 | 92 | 96:4 |
| 2 | 1b, R1= Me, R2 = H | 2b | 73 | 92 | 96:4 |
| 3 | 1c, R1= Me, R2 = Cl | 2c | 45 | 92 | 96:4 |
| 4 | 1d, R1= Me, R2 = OMe | 2d | 75 | 94 | 97:3 |
| 5 | 1e, R1= Ph, R2 = Me | 2e | 78 | 94 | 97:3 |
| 6 | 1f, R1 = -CH2(CH2)2CH2-, R2= Me | 2f | 83 | 92 | 96:4 |
| 7 | 1g, R1= -CH2(CH2)3CH2-, R2 = Me | 2g | 68 | 92 | 96:4 |
| 8 | 1h, R1= H, R2 = Me | 2h | 68 | 80 | 90:10 |
| 9 | 1i, R1 = H, R2= H | 2i | 77 | 82 | 91:9 |
| 10d |
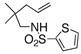
14 |
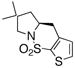
15 |
30 | 86 | 93:7 |
| 11 |
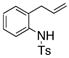
16 |
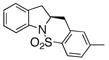
17 |
75 | 46 | 73:27 |
| 12d |
18 |
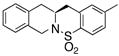
19 |
63 | 72 | 86:14 |
Reaction conditions: Cu(OTf)2 (0.2 equiv) and (R,R)-11a (0.2 equiv) were combined and treated with PhCF3 (0.1 M w/r to substrate) and heated at 50 °C for 1h. Substrate (1 equiv), MnO2 (3 equiv) and K2CO3 (1 equiv) were added and the reaction tube was sealed and heated at 120 °C for 24 h unless otherwise noted. All reactions were run at least two times to ensure reproducibility.
Yields refer to amount isolated after purification by flash chromatography on SiO2.
Enantiomeric excess and ratios were determined by chiral HPLC anlaysis (chiralcel OD-H or AD-RH).
Reactions were run for 96 h.
