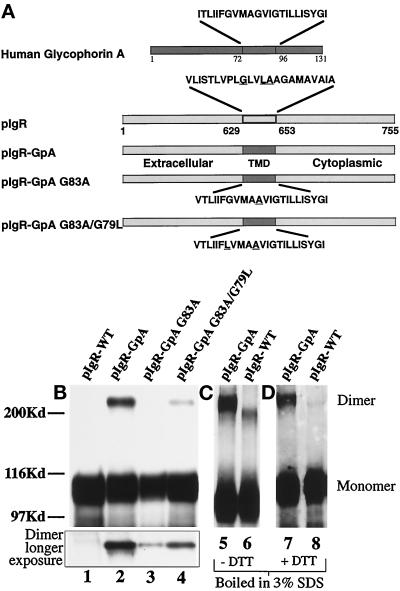
Figure 3.
Construction and expression of pIgR-GpA chimeras. (a) A schematic representation of the pIgR-WT, pIgR-GpA, pIgR-GpA-G83A, and pIgR-GpA-G83A/G79L. A unique restriction site was engineered into the pIgR at the 3′-end of the pIgR TMD. Oligonucleotides for the wild-type human glycophorin A TMD were generated with overhangs corresponding to the restriction sites ScaI and AscI. The mutations in the glycophorin TMD were created by PCR mutagenesis. Due to the 5′-restriction site, the first amino acid, Val, of the pIgR TMD was retained. The remaining amino acids correspond to the GpA TMD. Substitution of Val for Ile had no effect on the dimerization of GpA (Lemmon et al., 1992a). For the chimeric constructs, underlined amino acids represent those residues mutated. For the wild-type pIgR, the underlined residues are those corresponding to the dimerization motif described by Sternberg and Gullick (1990). (b) Detection of dimeric receptor by SDS-PAGE. MDCK cells transfected with pIgR-WT (lane 1), pIgR-GpA (lane 2), pIgR-GpA G83A (lane 3), and pIgR-GpA G83A/G79L (lane 4) constructs. Immunoprecipitates were analyzed by SDS-PAGE/Western blot (SC166 mouse monoclonal against pIgR cytoplasmic domain); 30 sec exposure by ECL. The inset represents the higher molecular weight dimer band at a longer exposure of the same gel. (c) Pellets of cells expressing pIgR-GpA (lane 5) or pIgR-WT (lane 6) were lysed with hot 3% SDS, boiled for 5 min, and then diluted 10-fold with 10 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, and 1% Triton X-100, before immunoprecipitation with sheep anti-SC conjugated to protein G and analyzed by SDS-PAGE minus DTT. (d) Pellets of cells expressing pIgR-GpA (lane 7) or pIgR-WT (lane 8) were processed as in panel c, except that the samples were reduced with DTT before electrophoresis. In panels c and d, pIgR was detected by Western blot with sheep anti-SC followed by secondary anti-sheep-HRP and ECL.