Abstract
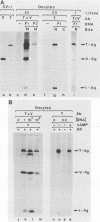
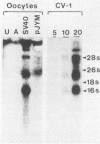
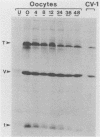
Simian virus 40 (SV40) large- and small-tumor antigens (T-Ag, t-Ag) are normally synthesized early after infection of either permissive (monkey) or nonpermissive (mouse) fibroblasts, whereas an equivalent amount of viral coat protein (V-Ag) is observed late after infection of permissive cells and only after viral DNA replication has occurred. To determine whether or not expression of these genes is regulated in the same manner during early mammalian development, SV40 DNA was injected into the nuclei of mouse oocytes and one- and two-cell embryos. In oocytes, about three times more V-Ag was produced than T-Ag, and both were synthesized concomitantly in the same cells. Viral mRNA and proteins synthesized in oocytes comigrated during gel electrophoresis with the same products synthesized in SV40-infected monkey cells. Viral gene expression required circular DNA molecules injected into the nuclei of transcriptionally and translationally active cells. Injected DNA was stable and underwent conformational changes consistent with chromatin assembly. Oocytes did not replicate either polyomavirus or SV40 DNA. Thus, the temporal order of viral gene expression is circumvented in mouse germ cells, allowing these proteins to be expressed concurrently and in equivalent amounts with no requirement for DNA replication. However, in preimplantation embryos, neither T-Ag nor V-Ag was detected by immunoprecipitation although T-Ag synthesis was demonstrated as a specific requirement for SV40 DNA replication. Thus, viral gene expression in mouse embryos as early as the one-cell stage was reduced at least 500-fold relative to that in oocytes. Similarities between SV40 gene expression in mouse oocytes and that in Xenopus oocytes suggest that germ cells in higher animals share common regulatory mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramczuk J., Vorbrodt A., Solter D., Koprowski H. Infection of mouse preimplantation embryos with simian virus 40 and polyoma virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):999–1003. doi: 10.1073/pnas.75.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendig M. M., Williams J. G. Fidelity of transcription of Xenopus laevis globin genes injected into Xenopus laevis oocytes and unfertilized eggs. Mol Cell Biol. 1984 Oct;4(10):2109–2119. doi: 10.1128/mcb.4.10.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., May E., Salzman N. P. Characterization of simian virus 40 tsA58 transcriptional intermediates at restrictive temperatures: relationship between DNA replication and transcription. J Virol. 1977 Jun;22(3):702–710. doi: 10.1128/jvi.22.3.702-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brady J., Khoury G. trans Activation of the simian virus 40 late transcription unit by T-antigen. Mol Cell Biol. 1985 Jun;5(6):1391–1399. doi: 10.1128/mcb.5.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Fromm M., Berg P. Complex regulation of simian virus 40 early-region transcription from different overlapping promoters. Mol Cell Biol. 1984 Sep;4(9):1900–1914. doi: 10.1128/mcb.4.9.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Rigby P. W. Fate of viral DNA in nonpermissive cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6638–6642. doi: 10.1073/pnas.78.11.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Gheysen D., Knowland J., van de Voorde A., Fiers W. Evidence for the direct involvement of DNA replication origin in synthesis of late SV40 RNA. Nature. 1982 Dec 9;300(5892):500–505. doi: 10.1038/300500a0. [DOI] [PubMed] [Google Scholar]
- Ernoult-Lange M., May E. Evidence of transcription from the late region of the integrated simian virus 40 genome in transformed cells: location of the 5' ends of late transcripts in cells abortively infected and in cells transformed by simian virus 40. J Virol. 1983 Jun;46(3):756–767. doi: 10.1128/jvi.46.3.756-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin L. D. Analysis of the mechanisms involved in gene regulation and cell differentiation by microinjection of purified genes and somatic cell nuclei into amphibian oocytes and eggs. Differentiation. 1982 May;21(3):149–159. doi: 10.1111/j.1432-0436.1982.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Gargiulo G., Razvi F., Worcel A. Assembly of transcriptionally active chromatin in Xenopus oocytes requires specific DNA binding factors. Cell. 1984 Sep;38(2):511–521. doi: 10.1016/0092-8674(84)90506-3. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Cabral F. Purification and characterization of a transformation-dependent protein secreted by cultured murine fibroblasts. Biochemistry. 1981 Mar 17;20(6):1659–1665. doi: 10.1021/bi00509a039. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulatory function of simian virus 40 DNA replication for late viral gene expression. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4831–4834. doi: 10.1073/pnas.74.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulatory mechanism of simian virus 40 gene expression in permissive and in nonpermissive cells. J Virol. 1976 Mar;17(3):854–858. doi: 10.1128/jvi.17.3.854-858.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Weintraub H., McKnight S. L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983 Mar 3;302(5903):38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Hartzell S. W., Byrne B. J., Subramanian K. N. The simian virus 40 minimal origin and the 72-base-pair repeat are required simultaneously for efficient induction of late gene expression with large tumor antigen. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6335–6339. doi: 10.1073/pnas.81.20.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Roberts B. E., Pratt D. M., David J. R., Miller J. S. Structure and arrangement of the beta-tubulin genes of Leishmania tropica. Mol Cell Biol. 1984 Jul;4(7):1372–1383. doi: 10.1128/mcb.4.7.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Analysis of an activatable promoter: sequences in the simian virus 40 late promoter required for T-antigen-mediated trans activation. Mol Cell Biol. 1985 Aug;5(8):1859–1869. doi: 10.1128/mcb.5.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick J. J., Stacey D. W. Differences in intracellular DNA ligation after microinjection and transfection. Mol Cell Biol. 1984 Feb;4(2):240–246. doi: 10.1128/mcb.4.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M. Organization and transcription of the simian virus 40 genome. Curr Top Microbiol Immunol. 1979;87:43–172. doi: 10.1007/978-3-642-67344-3_3. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Mertz J. E. Linear DNA does not form chromatin containing regularly spaced nucleosomes. Mol Cell Biol. 1982 Dec;2(12):1608–1618. doi: 10.1128/mcb.2.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli T., Prives C. Regulation of simian virus 40 gene expression in Xenopus laevis oocytes. Mol Cell Biol. 1985 Aug;5(8):2019–2028. doi: 10.1128/mcb.5.8.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Mertz J. E. Template structural requirements for transcription in vivo by RNA polymerase II. Mol Cell Biol. 1982 Dec;2(12):1595–1607. doi: 10.1128/mcb.2.12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Stephens D. L., Mertz J. E. Kinetics of accumulation and processing of simian virus 40 RNA in Xenopus laevis oocytes injected with simian virus 40 DNA. Mol Cell Biol. 1982 Dec;2(12):1581–1594. doi: 10.1128/mcb.2.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Wassarman P. M. Meiotic maturation of the mammalian oocyte in vitro: effect of dibutyryl cyclic AMP on protein synthesis. J Exp Zool. 1974 Aug;189(2):275–281. doi: 10.1002/jez.1401890217. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M., Josefowicz W. J. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978 May;156(2):209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Gurdon J. B. Post-transcriptional processing of simian virus 40 late transcripts in injected frog oocytes. J Mol Biol. 1983 Jan 5;163(1):1–26. doi: 10.1016/0022-2836(83)90027-x. [DOI] [PubMed] [Google Scholar]
- Wirak D. O., Chalifour L. E., Wassarman P. M., Muller W. J., Hassell J. A., DePamphilis M. L. Sequence-dependent DNA replication in preimplantation mouse embryos. Mol Cell Biol. 1985 Nov;5(11):2924–2935. doi: 10.1128/mcb.5.11.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]