Abstract
Functional maturation of the small intestine occurs during the weaning period in rats. It is known that this development is facilitated by glucocorticoid. However, the effect of glucocorticoid on morphological development of small intestine has yet to be clarified. The present study evaluated the morphological development and cell proliferation of the small intestine in adrenalectomized (ADX) rat pups. To further understand the mechanism of glucocorticoid effects on intestinal development, we examined the localization of the glucocorticoid receptor in the small intestine. Microscopic analysis showed that growth of villi and crypts is age-dependent, and is significantly attenuated in ADX rats compared with sham-operated rats. BrdU-positive cells, i.e. proliferating cells, were primarily observed in crypt compartments and rapidly increased in number during the early weaning period. The increase in BrdU-positive cells could be attenuated by adrenalectomy. The morphological development of small intestine may be associated with increased proliferation of epithelial cells. On the other hand, glucocorticoid receptors were found in epithelial cells of the mid- and lower villi and not in crypts where BrdU-positive cells were localized. These results indicate that the growth of small intestine is attenuated by adrenalectomy, and that glucocorticoid indirectly acts on proliferation of epithelial cells during the weaning period.
Keywords: glucocorticoid, intestinal development, weaning
I. Introduction
The small intestine of rat pups is immature at birth. However, it dramatically develops during the third postnatal week, about 18 days after birth when pups begin to ingest solid food. Villi of intestine expand from a finger-like to a leaf-like shape, and the crypts extend [4, 23]. Additionally, the activity of digestive enzymes such as sucrase and maltase rise to the adult level by the age of four weeks when pups subsist on solid food [20]. Morphological and functional development of the intestine occurs during the weaning period, which is considered to be adaptive for this dietary transition.
It has been suggested that glucocorticoids play a critical role in intestinal development during the weaning period. Henning and collaborators have shown that administration of a synthetic glucocorticoid, dexamethasone, increased the activities of sucrase, trehalase and glucoamylase in the small intestine of weaning rats [11, 13, 14]. When rats were adrenalectomized (ADX), the activities of sucrase and maltase decreased, and those of lactase and acid β-galactosidase needed for milk digestion increased [12, 14]. Administration of corticosterone to ADX rats restored the activities of sucrase and maltase enzymes [12]. In addition, administration of dexamethasone increased expression of sucrase-isomaltase mRNA and trehalase mRNA in the small intestine [11, 22]. Thus, glucocorticoid appears to facilitate intestinal maturation by affecting gene transcription. It is known that glucocorticoid action occurs through the glucocorticoid receptor. This receptor functions as a transcription factor to regulate the expression of target genes. Recent studies have attempted to determine the functional development of intestine mediated by glucocorticoid at the genetic level [1, 15].
On the other hand, the mechanism of how glucocorticoids affect morphological development of the small intestine during the weaning period is not known. The lumen of the small intestine is lined by a layer of epithelial cells. The intestinal epithelial cells proliferate and differentiate in the crypt and migrate to the top of the villus. The morphological development of small intestine may be associated with the proliferation of enterocytes. In this study, we studied the morphological changes and cell proliferation in the small intestine of ADX rats to elucidate the role of glucocorticoid on the morphological development in the small intestine during the weaning period. Further, to investigate the mechanism of action of glucocorticoid on intestinal development, we examined the localization and expression of glucocorticoid receptor in the small intestine of rat pups.
II. Materials and Methods
Animals and adrenalectomy
This study was approved by the Tamagawa University Animal Care and Use Committee and conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Wistar rats purchased from Clea Japan (Tokyo, Japan) were mated, and their litters served as subjects. A mother and her litter were housed in a plastic cage (28×44×18 cm), and were given laboratory chow (Oriental Yeast, Tokyo, Japan) and water ad libitum. The animal room was maintained on a 12:12 hr light-dark cycle, with lights on at 0700. Room temperature ranged from 20 to 24°C, and humidity was not controlled. Within two days of birth, litters were culled to 10 pups. At 12 days of age, five pups were adrenalectomized bilaterally via dorsal incision under pentobarbital anesthesia (25 mg/kg), and the others were sham-operated as a control. The numbers of male and female rats were equalized between adrenalectomized (ADX) and sham-operated rats. The rat pups were sacrificed at 16, 18, 20, 22, 24 and 26 days of age. For bromodeoxyuridine (BrdU) immunohistochemistry, rats were injected intraperitoneally with 45 mg/kg BrdU (Upstate Biotechnology, Lake Placid NY, USA) 1 hr before sacrifice. The small intestine was harvested. The trunk blood was also collected, and concentrations of plasma corticosterone were measured by HPLC [21].
Tissue preparation
A part of the jejunum was excised from ADX and sham-operated rats. The tissues were washed with phosphate-buffered saline (PBS) and cut into nearly straight cylinder-shaped segments of 10–15 mm in length. They were fixed in 3% formaldehyde solution overnight for hematoxylin and eosin staining and BrdU immunohistochemistry. Tissue samples for glucocorticoid receptor immunohistochemistry were immersed in periodate-lysine-paraformaldehyde fixative at 4°C for 6 hr. Then jejunal segments were embedded in paraffin and sectioned to 4 µm-thickness. The sections were mounted on silane-coated slides, treated with xylene to remove paraffin, and rehydrated by using decreasing concentrations of ethanol.
Morphological observations of the small intestine
The sections were stained with hematoxylin and eosin to visualize the morphological features. The stained sections were observed with a compound light microscope (Olympus, Tokyo, Japan). For measurement of villus area and crypt length, a fixed small fragment of central part of jejunum was stained with Feulgen reagent and microdissected [8]. The stained small segment of jejunum was divided into 5 pieces by perpendicular cuts across the intestine. These were placed on microscope slides, and images of the cross-sections were captured with a digital camera attached to the microscope. Two villus areas and crypt lengths were measured per cross-section and calculated using Image J software (NIH, http://rsb.info.nih.gov/ij/). Statistical analysis was performed by ANOVA and Student’s t-test.
BrdU immunohistochemistry
The sections were immersed in 0.3% H2O2 to inactivate endogenous peroxidase. Sections were denatured in 2 N HCl for 2 hr and were treated with 0.2% pepsin in 0.01 N HCl at 37°C for 15 min. After blocking with PBS containing 1% BSA and 0.5% Tween-20, the sections were incubated with anti-BrdU antibody (1/750, PROGEN Biotechnik, Heidelberg, Germany) for 1 hr. The sections were exposed to biotinylated anti-mouse IgG (Vector Laboratories, Burlingame CA, USA) for 1 hr followed by incubation with horseradish peroxidase (HRP)-streptavidin (Vector Laboratories) for 1 hr. Between each step, the sections were rinsed three times with PBS. The first incubation was with PBS containing 0.05% 3,3'-diamonobenzidine tetrahydrochloride (DAB; Dojin Chemicals, Kumamoto, Japan) and 0.03% H2O2. The sections were counterstained with hematoxylin solution. BrdU-positive cells in each crypt compartment were counted and expressed as the number of BrdU-positive cells in a crypt. Statistical analysis was performed by ANOVA and Student’s t-test.
Glucocorticoid receptor immunohistochemistry
The sections were heated in a standard microwaves oven twice for 5 min in 10 mM citrate buffer (pH 6.0), immersed in 0.3% H2O2 for 15 min, and incubated with 1% BSA/PBS blocking solution followed by anti-glucocorticoid receptor antibody (1/7,000, Santa Cruz Biotechnology, Santa Cruz CA, USA) at 4°C overnight, then with biotinylated anti-rabbit IgG (Vector Laboratories) for 3 hr, and finally with HRP-streptavidin (Vector Laboratories) for 1 hr. The sections were stained with PBS containing 0.05% DAB, 0.03% H2O2 and 1.5% nickel ammonium sulfate.
Western blot analysis of the glucocorticoid receptor
The epithelial cells of the jejunum were isolated as described previously [2]. Briefly, the 5 cm segments of the jejunum tissue were everted over an animal feeding needle, followed by incubation in PBS containing 3 mM EDTA at 37°C for 20 min. The medium containing the segments was vibrated for 10 sec, and the suspension of epithelial cells was collected into a tube and centrifuged at 300×g for 5 min. The pellets were resuspended in Hank’s balanced salt solution containing 5 mM EDTA and 1 mM PMSF. The epithelial cells in solution were homogenized with a Polytron homogenizer. The homogenates were centrifuged at 10,000×g for 30 min, and supernatants were collected. The protein concentrations of homogenates were measured by the Bradford method.
The intestinal homogenates (2.5 µg of protein content) were electrophoresed on a 7.5% polyacrylamide gel and transferred to PVDF membranes. After blocking with PBS containing 5% skim milk and 0.1% Tween-20, membranes were incubated in a blocking solution then in diluted anti-glucocorticoid receptor antibody (1/2,000, Santa Cruz Biotechnology) overnight, followed by incubation with HRP-linked secondary antibody (1/1,000, Cell Signaling Technology, Danvers MA, USA) for 1 hr. The membranes were reacted with ECL reagent (Amersham Bioscience, Little Chalfont, UK) and exposed to X-ray film.
III. Results
In the present study, we investigated the effects of adrenalectomy on morphological development and cell proliferation in rat small intestine during the weaning period. Furthermore, expression and localization of glucocorticoid receptor in the small intestine were determined. The plasma corticosterone concentration of adrenalectomized rat pups at 12 days of age was less than 20 ng/ml, which is 10–15% of that in the sham-operated rats. There was no difference in body weight or small intestinal weight between ADX and sham-operated rats.
Effect of adrenalectomy on morphological development of the small intestine
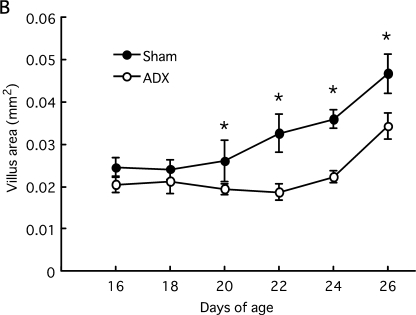
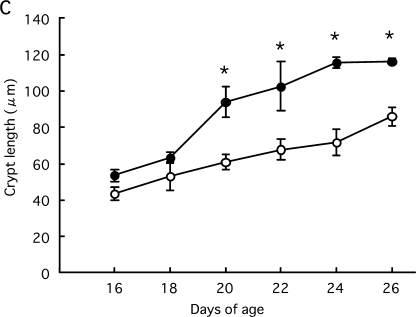
ADX and sham-operated rats at 16 and 18 days of age had finger-shaped villi. At day 20, the villus area increased and the villus became leaf-shaped in sham-operated rats. Crypt length also greatly increased from 18 to 20 days of age and gradually extended thereafter (Fig. 1A). In contrast, ADX rats had finger-shaped villi and exhibited no increase in the villus area until day 24. The growth of crypt length was attenuated compared to sham-operated rats. From 20 to 26 days of age the villus area and crypt length were significantly lower in ADX rats than sham-operated rates (Fig. 1B, C). Therefore, adrenalectomy attenuated the morphological development of small intestine.
Fig. 1.
Morphological development of small intestine in rats during the weaning period. Rat pups were adrenalectomized or sham-operated at 12 days of age. Small intestines were removed at 16, 18, 20, 22, 24 and 26 days of age. The formalin-fixed paraffin sections (4 µm) were stained with hematoxylin-eosin (A, Bar=100 µm). Intestinal fragments were stained with the Feulgen reaction and microdissected. The villus area (B) and crypt length (C) were measured under the microscope. Statistical analysis was performed by ANOVA and Student’s t-test (* p<0.05).
Effect of adrenalectomy on cell proliferation in the small intestine
The photomicrographs and transitions of BrdU-positive cells in small intestine are shown in Figure 2. The BrdU-positive cells were primarily located in the crypt, although a few cells were observed in the lamina propria. The BrdU-positive cells in the crypt represent proliferating epithelial cells. The number of these cells at the lower 75% of the crypt increased according to crypt elongation (Fig. 2A). These proliferating cells increased in sham-operated rats until 24 days of age. Adrenalectomy significantly decreased the number of BrdU-positive cells in crypts of the jejunum from 18 to 26 days after birth in comparison with sham-operated rats (Fig. 2B). A decrease in the number of BrdU-positive cells in ADX rats suggests that deletion of corticosterone led to a delay in the increase of proliferative cells.
Fig. 2.
BrdU-positive cells of small intestine in rats during the weaning period. Rat pups were adrenalectomized or sham-operated at 12 days of age. Rat were injected with BrdU and sacrificed at 16, 18, 20, 22, 24 and 26 days of age. The BrdU was detected in formalin-fixed paraffin sections by immunohistochemistry. BrdU labeling was stained with DAB (Brown), and counterstained with hematoxylin (A, Bar=100 µm). The BrdU-positive cells in the crypt compartment were counted and expressed as the number of BrdU-positive cells per crypt (B). Statistical analysis was performed by ANOVA and Student’s t-test (* p<0.05).
Localization and expression of glucocorticoid receptor in small intestine
Immunoreactivity of glucocorticoid receptors was observed in epithelial cells of the mid- and lower villus (Fig. 3A). The glucocorticoid receptors were localized in the nuclei of epithelial cells. Glucocorticoid receptor expression was absent in crypts and the lamina propria. During the whole weaning period the localization of glucocorticoid receptor did not change. There was no difference in the levels of expression of glucocorticoid receptors between ADX and sham-operated rats (Fig. 3B).
Fig. 3.
Localization and expression of glucocorticoid receptor in rat small intestine during the weaning period. Rat pups were adrenalectomized or sham-operated at 12 days of age. Small intestines were removed at 16, 18, 20, 22, 24 and 26 days of age. Localization of glucocorticoid receptor (GR) was examined on PLP-fixed paraffin sections by immunohistochemistry. GR was stained with Ni-DAB (Dark blue) (A, Bar=100 µm). The levels of expression of GR were determined by western blotting (B).
IV. Discussion
The results of this study indicate that the growth of villi and crypts in the small intestine was attenuated by adrenalectomy during the weaning period. In particular, villus area and crypt length hardly increased in the early weaning period and were significantly lower by late weaning. These morphological results are consistent with the effects of adrenalectomy on functional development as determined by decreases in sucrase activity and sucrase-isomaltase mRNA expression [14]. However, sucrase activity and sucrase-isomaltase expression increased to control level by 26 days of age although villus area and crypt length still differed between ADX and sham-operated rats at that time. These differences are attributed to the fact that the morphological development such as growth of villus and crypt begins after 20 days of age and continues until 72 days [7]. Thus, morphological growth occurs in late and post-weaning periods, and is later than functional development, although it is not clear whether villus area and crypt length in ADX rats increase to control levels in the post-weaning period.
The numbers of BrdU-positive cells in the intestines of ADX rats were low compared to those in sham-operated rats. Because the increase in BrdU-positive cells was prominent at early weaning in sham-operated rats, it is thought that adrenalectomy leads to a delay in the onset of increased proliferation. This early increase of proliferating cells was followed by growth of villi and crypts by late weaning. Since adrenalectomy decreased the number of BrdU-positive cells in crypts, it is possible that the glucocorticoid released from the adrenal cortex promotes proliferation of epithelial cells. However, it is known that glucocorticoid inhibits proliferation and induces apoptosis in many cell types [5, 24]. The anti-proliferative effects of glucocorticoid are induced through the glucocorticoid receptor by the transcriptional regulation of factors associated with the cell cycle. For example, there is the down-regulation of cyclin D1, cyclin-dependent kinase 4, c-myc and Bcl-2 [19, 24].
In this study, the localization and expression of glucocorticoid receptors were examined by immunohistochemistry and western blotting. The results show that the glucocorticoid receptors were localized in the epithelial cells of the mid- and lower villus and were absent from the crypt. The glucocorticoid receptors were lacking in proliferating cells of the crypt, and it is thought that the inhibition of proliferation is not elicited by glucocorticoid. Moreover, there was no difference in the expression of glucocorticoid receptors between ADX and sham-operated rats. The inhibitory influence on the increase of BrdU-positive cells in the crypt arises from deletion of glucocorticoid. Taking into consideration the localization of glucocorticoid receptors, we conjecture that glucocorticoid promotes the proliferation of epithelial cells and intestinal growth through other factors and mechanisms.
For many years, the roles of various growth factors on intestinal development during the suckling and weaning periods have been investigated [6]. For example, epidermal growth factor, insulin-like growth factor I and transforming growth factor-α stimulate cell proliferation in the crypts of the small intestine [9, 17]. The receptors for these growth factors are tyrosine kinases, which induce cell proliferation through Ras-MAP kinase and the phosphoinositol-3 kinase pathway. These growth factors are expressed in the intestine and other organs and are also contained in the milk of many mammalian species [18, 25, 26], suggesting that dam’s milk promotes intestinal development. Recently, it has been reported that the Wnt/β-catenin pathway is involved in the cell proliferation and differentiation of intestinal epithelium [10, 16]. Wnt signaling molecules, which include 19 proteins identified in humans and rodents, play critical roles in tissue patterning, cell fate and cell proliferation during embryonic and postnatal development. Wnt signaling is considered necessary for epithelial stem cell proliferation in intestine [3]. In addition, it has been reported that glucocorticoid-induced transcription of Wnt occurs in the intestine of mice [1]. Therefore, Wnt and other factors are candidates for mediator molecules of intestinal growth via glucocorticoids. Further studies are required to elucidate the mechanism of glucocorticoid-induced intestinal development.
In conclusion, our study provides evidence that the growth of the small intestine is impaired by adrenalectomy, and that glucocorticoid indirectly acts on proliferation of epithelial cells during the weaning period.
V. References
- 1.Agbemafle B. M., Oesterreicher T. J., Shaw C. A., Henning S. J. Immediate early genes of glucocorticoid action on the developing intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G897–G906. doi: 10.1152/ajpgi.00454.2004. [DOI] [PubMed] [Google Scholar]
- 2.Bjerknes M., Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat. Rec. 1981;199:565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- 3.Bjerknes M., Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G381–G387. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R. M. The effect of growth and of fasting on the number of villi and crypts in the small intestine of the albino rat. J. Anat. 1972;112:27–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Cook P. W., Swanson K. T., Edward C. P., Firestone G. L. Glucocorticoid receptor-dependent inhibition of cellular proliferation in dexamethasone-resistant and hypersensitive rat hepatoma cell variants. Mol. Cell. Biol. 1988;8:1449–1459. doi: 10.1128/mcb.8.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins A. G., Thompson F. M. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut. 2002;51:748–754. doi: 10.1136/gut.51.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummins A. G., Jones B. J., Thompson F. M. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig. Dis. Sci. 2006;51:718–723. doi: 10.1007/s10620-006-3197-9. [DOI] [PubMed] [Google Scholar]
- 8.Hasan M., Ferguson A. Measurement of intestinal villi in non-specific and ulcer-associated duodenitis: correlation between area of microdissected villus and villus epithelial cell count. J. Clin. Pathol. 1981;34:1181–1186. doi: 10.1136/jcp.34.10.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hormi K., Lehy T. Transforming growth factor-alpha in vivo stimulates epithelial cell proliferation in digestive tissues of suckling rats. Gut. 1996;39:532–538. doi: 10.1136/gut.39.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhnert F., Davis C. R., Wang H. T. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. U S A. 2005;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeper L. L., McDonald M. C., Heath J. P., Henning S. J. Sucrase-isomaltase ontogeny: Synergism between glucocorticoids and thyroxine reflects increased mRNA and no change in cell migration. Biochem. Biophys. Res. Commun. 1998;246:765–770. doi: 10.1006/bbrc.1998.8707. [DOI] [PubMed] [Google Scholar]
- 12.Martin G. R., Henning S. J. Enzymic development of the small intestine: are glucocorticoids necessary? Am. J. Physiol. 1984;246:G695–G699. doi: 10.1152/ajpgi.1984.246.6.G695. [DOI] [PubMed] [Google Scholar]
- 13.McDonald M. C., Henning S. J. Synergistic effects of thyroxine and dexamethasone on enzyme ontogeny in rat small intestine. Pediatr. Res. 1992;32:306–311. doi: 10.1203/00006450-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Nanthakumar N. N., Henning S. J. Ontogeny of sucrase-isomaltase gene expression in rat intestine: responsiveness to glucocorticoids. Am. J. Physiol. 1993;264:G306–G311. doi: 10.1152/ajpgi.1993.264.2.G306. [DOI] [PubMed] [Google Scholar]
- 15.Oesterreicher T. J., Henning S. J. Rapid induction of GATA transcription factors in developing mouse intestine following glucocorticoid administration. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G947–G953. doi: 10.1152/ajpgi.00470.2003. [DOI] [PubMed] [Google Scholar]
- 16.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Gene Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potten C. S., Owen G., Hewitt D., Chadwick C. A., Hendry H., Loed B. I., Woolford L. B. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut. 1995;36:864–873. doi: 10.1136/gut.36.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raaberg L., Nexo E., Mikkelson J. D., Poulsen S. S. Immunohistochemical localization and developmental aspects of epidermal growth factor in the rat. Histochemistry. 1988;89:351–356. doi: 10.1007/BF00500636. [DOI] [PubMed] [Google Scholar]
- 19.Rogatsky I., Trowbridge J. M., Garabedian M. J. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol. Cell. Biol. 1997;17:3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubino A., Zimbalatti F., Auricchio S. Intestinal disaccharidase activities in adult and suckling rats. Biochim. Biophys. Acta. 1964;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- 21.Sargent R. N. Determination of corticosterone in rat plasma by HPLC. J. Anal. Toxicol. 1985;9:20–23. doi: 10.1093/jat/9.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Solomon N. S., Gartner H., Oesterreicher T. J., Henning S. J. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr. Res. 2001;49:782–788. doi: 10.1203/00006450-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 23.St Clair W. H., Osborne J. W. Crypt fission and crypt number in the small and large bowel of postnatal rats. Cell Tissue Kinet. 1985;18:255–262. doi: 10.1111/j.1365-2184.1985.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 24.Thompson E. B., Thulasi R., Saeed M. F., Johnson B. H. Glucocorticoid antagonist RU 486 reverses agonist-induced apoptosis and c-myc repression in human leukemic CEM-C7. Annu. Rev. Biochem. 1995;63:261–275. doi: 10.1111/j.1749-6632.1995.tb31383.x. [DOI] [PubMed] [Google Scholar]
- 25.Xian C. J., Mardell C. E., Read L. C. Specificity of the localization of transforming growth factor-α immunoreactivity in colon mucosa. J. Histochem. Cytochem. 1999;47:949–957. doi: 10.1177/002215549904700712. [DOI] [PubMed] [Google Scholar]
- 26.Xu R. J. Development of the newborn GI tract and its relation to colostrum/milk intake: a review. Reprod. Fertil. Dev. 1996;8:35–48. doi: 10.1071/rd9960035. [DOI] [PubMed] [Google Scholar]