Table 3.
3-Methyl-5-arylfuran enol carbonate rearrangement
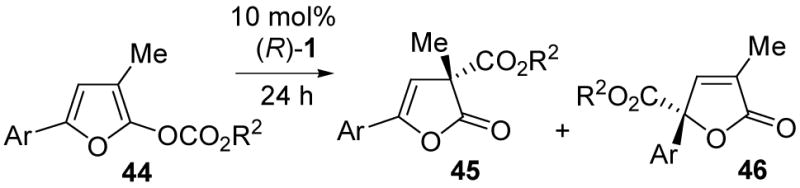 | ||||
|---|---|---|---|---|
| Entry | Substrate | Solvent | 45:46 a | eemajor (%) |
| 1b | 44a | CH2Cl2 | 3 : 2 | 75 |
| 2b | 44b | CH2Cl2 | 3 : 2 | 91 |
| 3 | 44c | CH2Cl2 | 5 : 1 | 82 |
| 4 | 44c | THF | 10 : 1 | 90c |
| 5 | 44c | Et2O | 11 : 1 | 83 |
| 6 | 44c | toluene | 10 : 1 | 74 |
| 7 | 44c | t-amyl-OH | 6 : 1 | 91 |
| 8d | 44d | CH2Cl2 | 1 : 4 | 63 |
| 9d | 44d | THF | 1 : 4 | 74 |
| 10d | 44d | Et2O | 1 : 4 | 90e |
| 11d | 44d | toluene | 1 : 4 | 83 |
| 12d | 44d | t-amyl-OH | 1 : 4 | 50 |
ratio based on NMR assay, >95% conversion
1 mol% of catalyst 1
45: 83% yield 45; 46: 7% yield (80% ee)
reaction complete in 4 h
46: 75% yield; 45: 25% yield
