Figure 5.
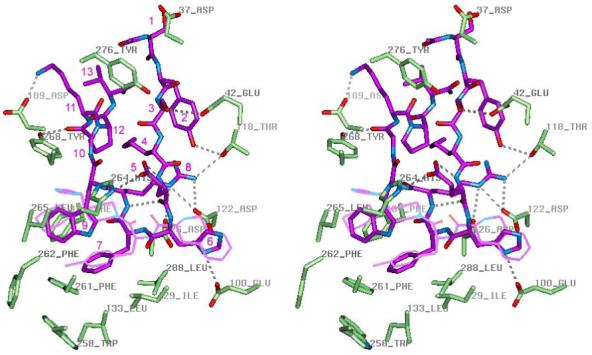
Stereoview of peptide agonist NDP-MSH (purple) inside the binding pocket of the hMC4R active conformation model. The mutated hMC4R residues and residues forming H-bonds with peptide are shown in the licorice representation colored by element. THIQ is shown in thin purple line for comparison. The receptor-bound conformation of NDP-MSH represents a β-hairpin-like structure with a reverse turn spanning His6 and D-Phe7, aromatic rings of D-Phe7 and Trp9 forming stacking interactions, and polar residues Glu5 and Arg8 located on different sides of the β-hairpin. Inside the binding pocket, NDP-MSH forms at least nine H-bonds with polar groups of the receptor (shown by dashed lines): Tyr2 hydroxyl with Thr118 hydroxyl, carboxylate group of Asp5 and backbone carbonyl of Trp9 with His264, imidazole of His6 with Glu100, guanidinium group of Arg8 with Asp122 and Asp126 carboxylate groups and hydroxyl of Thr118, backbone amide group of D-Phe7 with Asp126 carboxylate, backbone carbonyl of Gly10 with Tyr268 hydroxyl, and amine group of Lys11 with Asp189 carboxylate. Important hydrophobic interactions are formed between aromatic rings of D-Phe7 and Trp9 of peptide agonist and non-polar binding pocket residues , Ile129, Leu133, Phe184, Leu265, Trp258, Phe261, Leu288.
