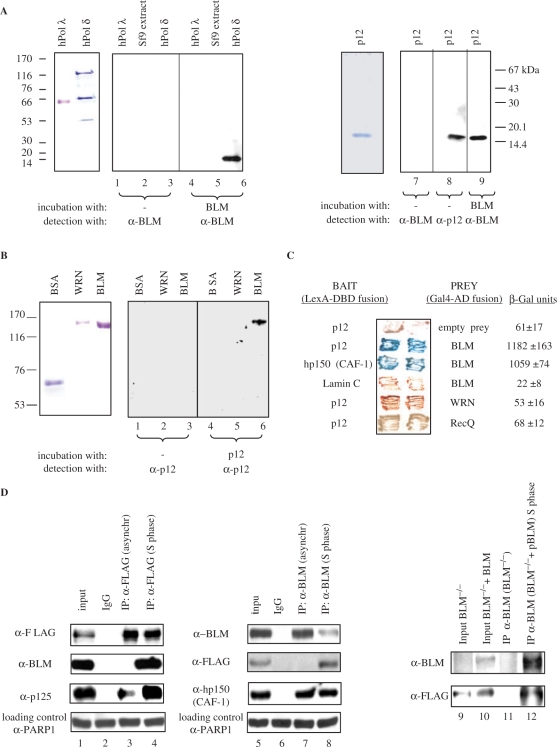
Figure 1.
BLM and p12 of hPOL δ interact in vitro and in vivo. (A) Left panel, far-western analysis. hPOL λ, total protein extract from Sf9 insect cells, and hPOL δ enzyme were subjected to SDS–PAGE, transferred to a nitrocellulose membrane, and were incubated with purified recombinant BLM. Anti-BLM antibodies were used to permit the detection of p12 as a novel BLM-interacting protein (lane 6). Molecular weight markers are also indicated on the left. Right panel, purified p12 subunit of hPOL δ was subjected to SDS–PAGE, transferred to nitrocellulose membrane, incubated with BLM, and subsequently probed with the anti-BLM antibody (lane 9). (B) Reciprocal far-western analysis. BSA, WRN and BLM (left panel) were hybridized with the purified p12 and probed with the anti-p12 antibody (right panel). Anti-p12 antibodies were used to confirm that p12 specifically binds to BLM (lane 6). (C) BLM and p12 of hPOL δ interact in the YTH assay. The L40 yeast reporter strain was co-transformed with plasmids encoding the indicated full-length ‘bait’ (LexA-DBD) and ‘prey’ (Gal4-AD) fusions. Two independent colonies were grown on SD agar plates lacking tryptophan and leucine, but containing X-gal, prior to assessment of β-galactosidase activity. Also shown are two negative controls, p12 co-transformed with an empty prey vector and BLM co-transformed with lamin C protein. The previously described BLM/hp150 (CAF-1) interaction (23) was used as a positive control. (D) BLM and hPOL δ form a complex in human cells. 293T cells were transiently transfected with FLAG-p12, and were synchronized in S phase using 1 mM HU. Nuclear extracts derived from either unsynchronized (lane 3) or S-phase synchronized cells (lane 4) were immunoprecipitated with the anti-FLAG antibody or control IgG, and were analysed by SDS–PAGE. One-tenth (50 µg) of the same nuclear extract was used as input control (lane 1). Immunoprecipitated FLAG-p12 and BLM were detected by western blotting using the anti-FLAG and anti-BLM antibody, respectively (lane 4). p125, the largest subunit of hPOL δ, was also efficiently co-immunoprecipitated using the same anti-FLAG antibody (lanes 3 and 4). Reciprocal co-IP is shown in the middle panel: lane 5, input; lane 6, IP with the control IgG; lane 7, IP with an anti-BLM antibody (C-18) from nuclear extracts derived from unsynchronized 293T cells; lane 8, IP with an anti-BLM antibody (C-18) from nuclear extracts derived from the S-phase synchronized 293T cells. The known BLM interacting protein, hp150 (CAF-1) was also efficiently co-immunoprecipitated using the same anti-BLM antibody (lanes 7 and 8). As a loading control for lanes 1–8, 50 µg of the corresponding nuclear extract was probed with an anti-PARP1 antibody. Right panel shows co-IP with anti-BLM antibody (C-18) from BS cell nuclear extracts (BS) and BS cells containing the BLM cDNA (BS + pBLM). p12 could be co-immunoprecipitated in the presence of BLM from the S-phase synchronized nuclear extracts (lane 12) but not in the absence of BLM (lane 11). Lanes 9 and 10 are the inputs of the two different nuclear extracts.