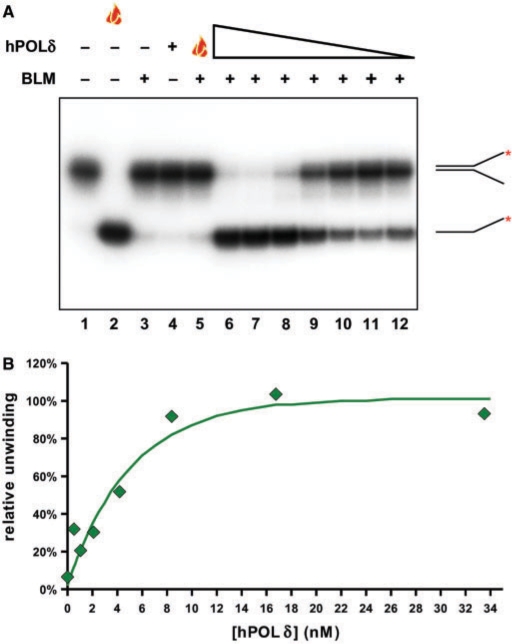
Figure 3.
The hPOL δ enzyme specifically stimulates the BLM-mediated unwinding of the replication fork substrate in a concentration-dependent manner. (A) A total of 1.3 nM BLM was pre-incubated with the exonuclease-defective hPOL δ enzyme in various concentrations (33.5, 16.8, 8.4, 4.2, 2.1, 1 and 0.5 nM; lanes 6–12, respectively) on ice for 3 min, and the samples were then warmed to 37°C. The unwinding reaction was initiated immediately by the addition of substrate and ATP. Flame symbol depicts heat-denatured substrate (lane 2), or BLM incubated with heat-denatured hPOL δ enzyme at the highest concentration of the titration range (33.5 nM; lane 5), as described earlier. (B) Quantification of data presented in (A). Data were normalized to the non-treated (lane 1, taken as 0%) and boiled (lane 2, taken as 100%) samples. Maximal stimulation (at 6 times above BLM basal helicase activity) was achieved with a 13 × molar excess of hPOL δ.