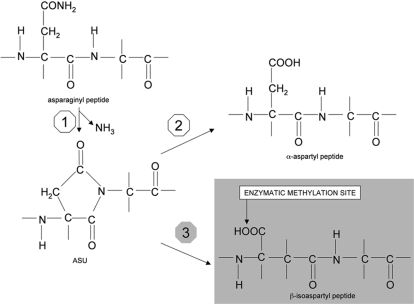
Figure 1. Mechanism for deamidation of asparaginyl residues in peptides.
(Step 1): the nitrogen of the Asn+1 residue (a Gly in the example) attacks the β-carbonyl carbon of the Asn, thus forming the succinimidyl derivative of the peptide (ASU) with the ammonia elimination. The ASU ring can open spontaneously on either side of the nitrogen atom. In one case the α-aspartyl peptide is formed (Step 2). In the other case the β-isoaspartyl peptide does occur (Step 3).