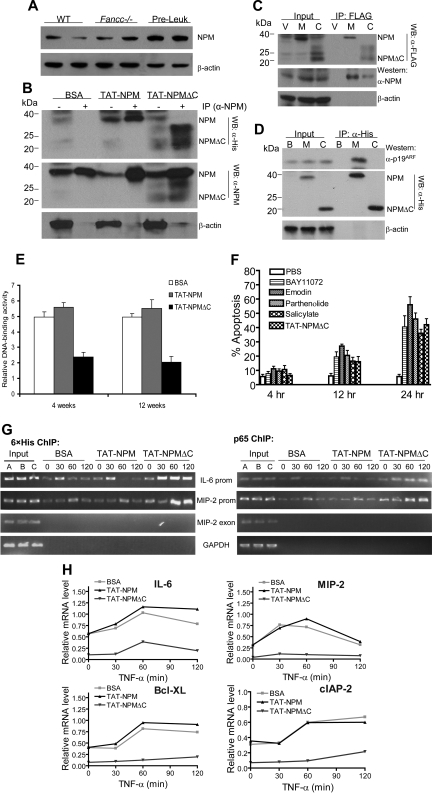
Figure 7.
TAT-NPMΔC forms complexes with endogenous NPM and p65 and abrogates NF-κB activation in Fancc−/− preleukemic cells. (A) Up-regulation of NPM in Fancc−/− preleukemic cells. Whole cell extracts of freshly isolated bone marrow cells from WT and Fancc−/− mice or Fancc−/− preleukemic cells were analyzed for NPM protein levels by immunoblotting. Data with 2 mice from each group are shown. (B) Nuclear association of TAT-NPM fusions with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes. Nuclear extracts were prepared and used for IP with an anti-NPM antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against 6 × histine (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (C) Nuclear association of NPMΔC with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were infected with vector (V), WT NPM (M), or NPMΔC (C) retroviruses. Nuclear extracts were prepared and used for IP with an anti-FLAG antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against FLAG (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lanes. (D) TAT-NPMΔC does not associate with p19ARF. Fancc−/− preleukemic cells were treated with BSA (B), TAT-NPM (M), or TAT-NPMΔC (C) (30 μg/mL each) for 30 minutes. Whole cell extracts were prepared and used for IP with an anti-His antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against p19ARF (top panel), 6 × histine (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (E) TAT-NPMΔC inhibits NF-kB activity in Fancc−/− leukemic mice. 106 Fancc−/− preleukemic cells (along with 106 competitive cells) were injected intravenously into lethally irradiated recipients, which after 10 days were injected intraperitoneally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for 4 and 12 weeks. Nuclear extracts were then prepared from BM cells of the recipient mice 24 hours after the last injection and DNA-binding activity of NF-κB was measured by transcription factor ELISA assays. The results are the means plus or minus SD of 2 independent experiments, each with 3 recipient mice from each group. Statistical significance (P < .05) between BSA or TAT-NPM and TAT-NPMΔC. (F) Kinetics of apoptosis induced by TAT-NPMΔC other NF-κB inhibitors in Fancc−/− leukemic cells. Fancc−/− preleukemic cells were treated with TAT-NPMΔC (30 μg/mL), sodium salicylate (5 mM), emodin 3 μg/mL), parthenolide (2 μM), or BAY11072 (0.2 μM) for the indicated time. Apoptosis was determined by flow cytometry for the percentage of cells with active caspase 3. The results are presented as the means plus or minus SD of 2 experiments. There is no statistically significant difference between TAT-NPMΔC and other tested NF-κB inhibitors. (G) Increased binding of TAT-NPMΔC and p65 to NF-κB–responsive promoters in Fancc−/− preleukemic cells. Chromatin immunoprecipitation assays. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). Recruitment of TAT-NPMΔC or the p65 NF-κB subunit to the IL-6 and MIP-2 promoters was assessed by immunoprecipitation and PCR amplification of the promoter sequences. (H) Down-regulation of proinflammatory and antiapoptotic genes in TAT-NPMΔC–treated Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). RNA was isolated, and gene expression was quantified by real-time PCR and normalized to the level of GAPDH mRNA.