Table 3.
Hydrosilylation of internal alkynes with complex 7.a
| entry | alkyne | number | silane | major product | number | ratiob | yieldc |
|---|---|---|---|---|---|---|---|
| 1 |
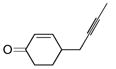
|
44 | (EtO)3SiH |
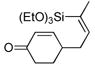
|
45 | 5:1 | 86 |
| 2 |
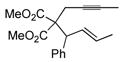
|
46 | Et3SiH |
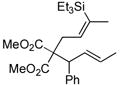
|
47 | >20:1 | 70d |
| 3 |
|
48 | (EtO)3SiH |
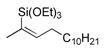
|
49 | 2.4:1 | 100 |
| 4e |
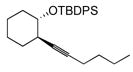
|
50 | (EtO)3SiH |
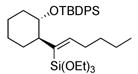
|
51 | >20:1 | (40% conv.) |
| 5e |
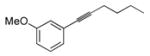
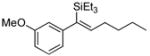
|
52 | Et3SiH |
|
53 | >20:1 | (20% conv.) |
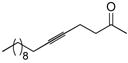
| 6 |
|
54 | (EtO)3SiH |
|
55 | 1:1 | 96g |
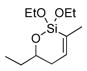
| 7 |
|
56 | (EtO)3SiH |
|
57 | 5:1 | 71d |
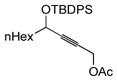
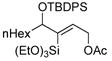
| 8f |
|
58 | (EtO)3SiH |
|
59 | 6:1 | 92 |
| 9 |
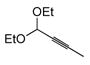
|
60 | (EtO)3SiH |
|
61 | >10:1 | 67g |
All reactions performed 0.5 M in CH2Cl2 on 0.2–0.5 mmol scale. 1.2 eq silane and 1 mol% complex 7 is employed unless otherwise indicated. Reactions were generally complete within 1 h.
Ratio of major:minor regioisomers determined by NMR and/or GC analysis of the crude reaction mixture. Only trans addition products were observed.
Isolated yield is for the mixture of regioisomers unless otherwise indicated.
Yield refers to isolated pure major product.
10 mol% catalyst loading.
4 mol% catalyst loading.
Product isolated as trans olefin after protodesilylation. See reference 14b.
