Table 5.
Hydrosilylation of alkynyl carbonyl compounds.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | alkyne | cmpd | silane | mol% 7 | product | yield | selectivityb |
| 1 |
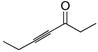
|
77 | BnMe2SiH | 0.5 | 78 | 98 | >20:1 |
| 2 | 77 |
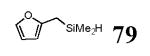
|
5 | 80 | 89 | >20:1 | |
| 3 |
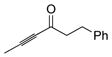
|
81 | (allyl)Me2SiH | 4 | 82 | 81 | >20:1 |
| 4 |
|
84 | PhMe2SiH | 2 | 85 | 96 | >20:1 |
| 5 | 84 | (EtO)3SiH | 1 | 86 | 99 | 5:1 | |
| 6 |
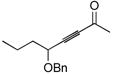
|
87 | BnMe2SiH | 1 | 88 | 67 (89)c | >20:1 |
| 7 |
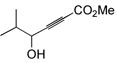
|
89 | BnMe2SiH | 1 | 90 | 88 | 7:1 |
| 8 |
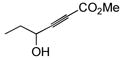
|
91 | (allyl)Me2SiH | 5 | 92 | 72 | 15:1 |
| 9 |
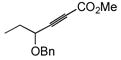
|
93 | BnMe2SiH | 3 | 94 | 34 | >20:1 |
| 10 |
|
95 | BnMe2SiH | 1 | 96 | 94 | >20:1 |
Reactions performed using 1.2 eq. silane at 0.5 M in acetone for 30 min.
Ratio of β-vinylsilane: α-vinylsilane determined by analysis of crude 1H NMR. In all cases only (Z)-vinylsilane isomers were observed.
Yield in parenthesis is based on recovered alkyne.
