Figure 1.
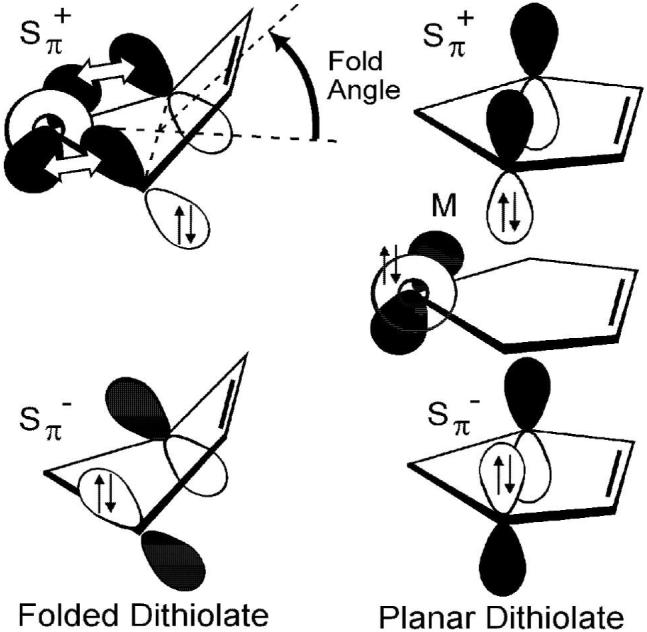
Representations of the valence orbitals of folded versus flat dithiolates. In the d0 case the metal orbital (M) is empty, and the Sπ+ orbital can interact upon folding. In the d2 case the metal orbital (M) is filled, and a planar orientation minimizes a filled-filled interaction. The Sπ- orbital does not have the correct symmetry to interact with the metal orbital.
