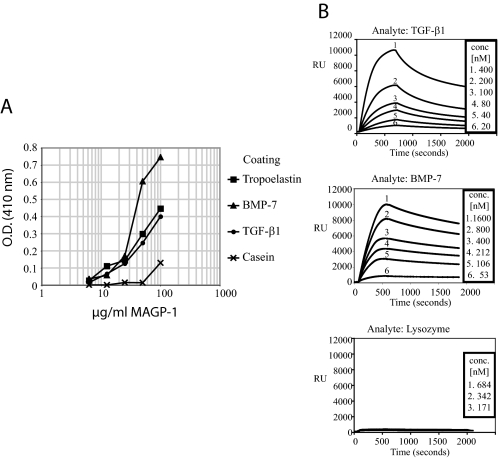
FIGURE 9.
MAGP-1 interacts with active TGF-β and BMP-7. A, solid phase binding assay demonstrating an interaction between MAGP-1 and active TGF-β and BMP-7. The growth factors were coated onto wells of a microtiter plate and incubated with dilutions of recombinant MAGP-1. Bound MAGP-1 was detected using a MAGP-1-specific antibody after thorough washing to remove unbound protein. Wells coated with casein and tropoelastin served as negative and positive controls, respectively. B, surface plasmon resonance experiments with immobilized MAGP-1 and BMP-7 and TGF-β1 in solution. Sensorgrams show binding of each growth factor at various concentrations to MAGP-1. TGF-β1 interacted with MAGP-1 with an affinity of 19.9 ± 6 nm (S.E., n = 16) and a dissociation rate of 4.0 × 10-3 (1/s) (C, top). BMP-7 bound to MAGP-1 with a KD of 10.5 ± 4.3 nm (S.E., n = 13) (C, middle). The dissociation rate constant was 2.6 × 10-4 (1/s). Lysozyme showed no measurable interaction with the MAGP-1-coated sensor chip (C, bottom).