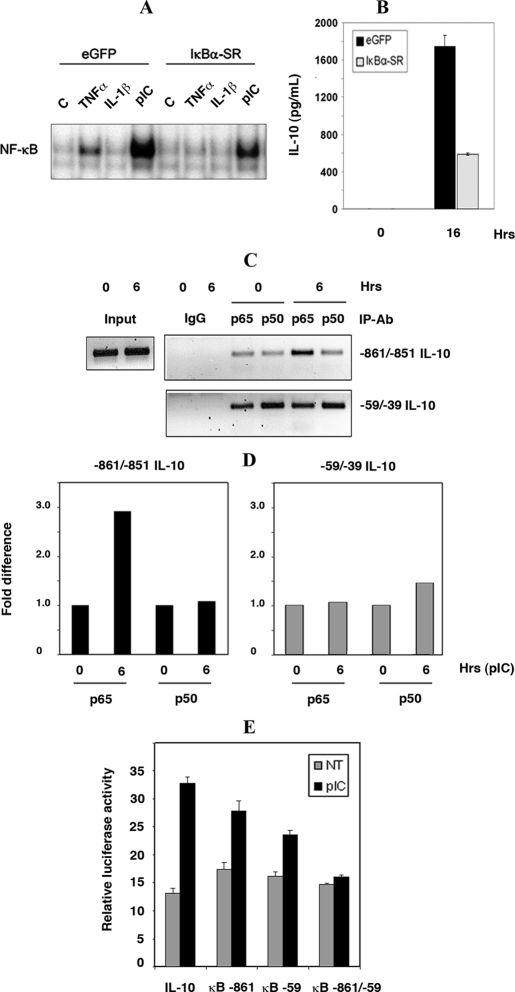
FIGURE 3.
dsRNA-mediated NF-κB-dependent regulation of IL-10 at a novel distal site on the gene promoter. A, direct activation of NF-κB was measured by EMSA in whole cell lysates (20 μg) from SM cells stably expressing IκBα-SR or GFP. Cells were untreated (C), or stimulated with pIC (50 μg/ml), and as controls TNFα (25 ng/ml), or IL-1β (5 ng/ml) for 30 min. B, role of NF-κB in dsRNA induction of IL-10 was verified by comparing IL-10 protein levels in supernatants from SM cells either stably expressing the IκBα-super-repressor (IκBα-SR) or, as a control, GFP (eGFP), either untreated or treated with pIC (50 μg/ml) for 16 h. C, binding of NF-κB to specific elements within the IL-10 promoter was measured by ChIP analysis. Protein complexes were immunoprecipitated with anti-p65, or anti-p50 antibodies from SM cell lysates either untreated or stimulated with pIC (50 μg/ml) for 6 h. Two regions of the IL-10 promoter, surrounding regions –59/–39 and –861/–851, were amplified from DNA obtained from the ChIP. As a positive control amplification of regions surrounding the α-amylase promoter is shown using input DNA before ChIP (Input). The specificity of the ChIP assay was tested using rabbit IgG. D, the difference in NF-κB binding to the proximal (–59/–39) or distal (–861/–851) sites in the IL-10 promoter was measured by quantitative PCR. Measures were normalized to the zero time point for each site. E, the functionality of each NF-κB binding site within the murine IL-10 promoter was tested by transient transfection of RAW264.7 cells with pGL3B-IL-10-LUC that were either wild-type (IL-10), mutated at the distal site (κB –861), or proximal site (κB –59), and at both sites (κB –861/–59).