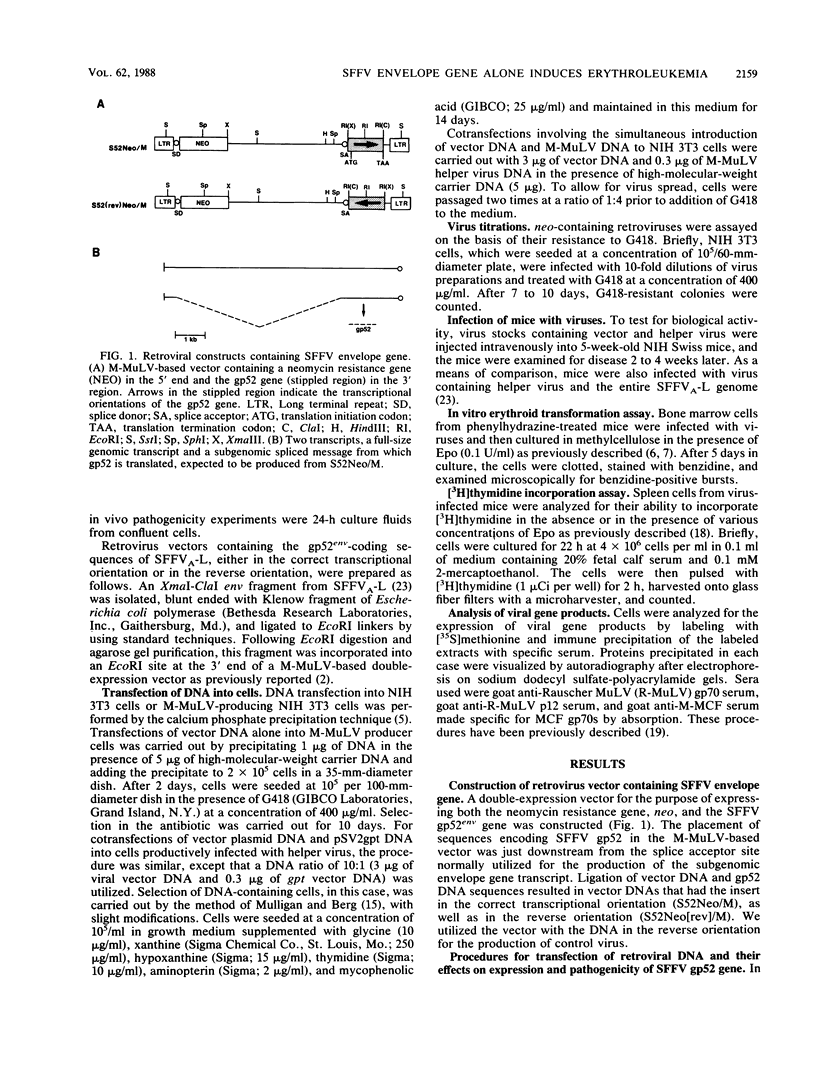
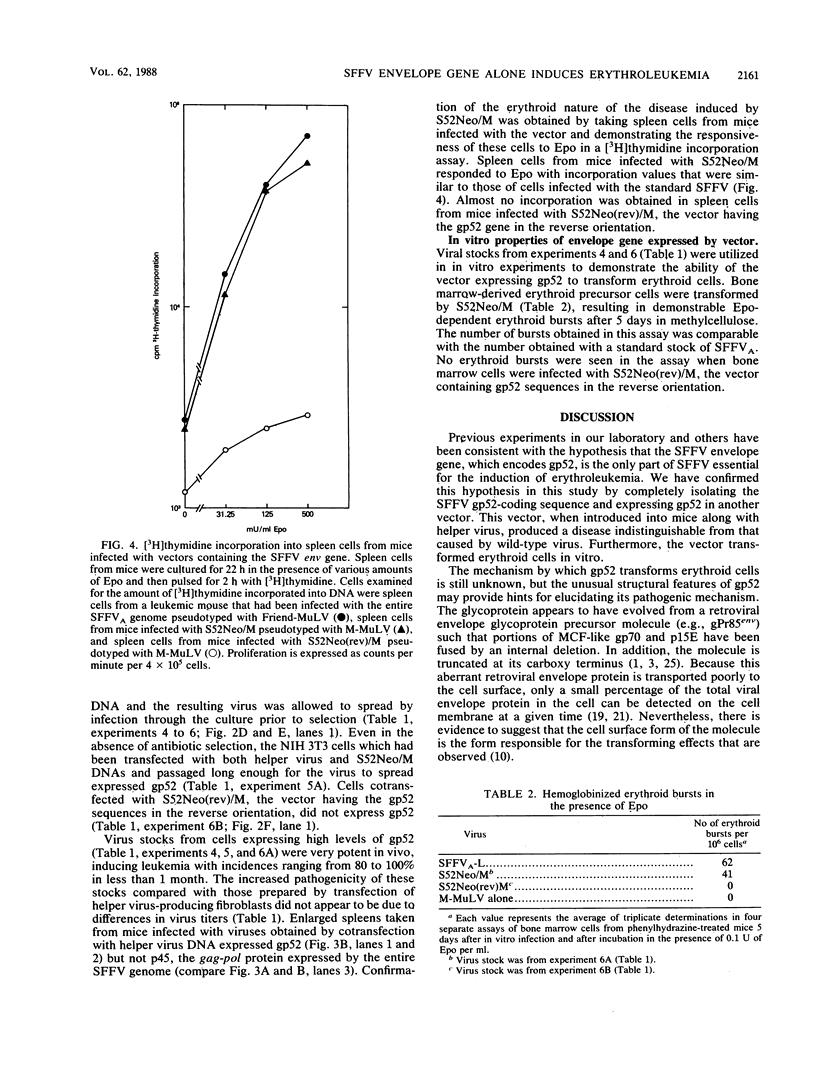
Abstract
Previous studies in our laboratory and others have been consistent with the hypothesis that the envelope (env) gene of the spleen focus-forming virus (SFFV) is the only gene essential for the induction of acute erythroleukemia. However, no studies have been carried out with the SFFV env gene in the complete absence of other SFFV sequences. To accomplish this goal, we isolated the sequences that encode the envelope glycoprotein, gp52, of SFFVA and expressed them in a Moloney murine leukemia virus-based double-expression vector containing the neomycin resistance gene. The method used to produce retrovirus stocks in tissue culture cells affected the expression of the gp52 gene in the vector and the subsequent pathogenicity of the vector in mice. Highly pathogenic virus stocks were obtained by cotransfection of vector and helper virus DNAs into fibroblasts, followed by virus replication and spread through the cell population. Mice infected with this stock developed a rapid erythroid disease that was indistinguishable from that induced by the entire SFFV genome, and the virus stock transformed erythroid cells in vitro. Spleen cells from the diseased mice expressed the SFFV env gene product but not the SFFV gag gene product. As expected, mice given the virus containing the SFFV env gene in the reverse orientation did not express the SFFV env gene product or develop erythroleukemia. This study, therefore, demonstrated (i) that double-expression retroviral vectors can be used under specific conditions to produce viruses expressing high levels of a particular gene and (ii) that incorporation of the SFFV env gene into such a vector in the absence of other SFFV sequences results in a retrovirus which is as pathogenic as the original SFFV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986 Dec;6(12):4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Krantz S. B. In vitro expression of erythroid differentiation induced by Friend polycythaemia virus. Nature. 1975 Feb 27;253(5494):731–732. doi: 10.1038/253731a0. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Hwang L. H., Gilboa E. Expression of genes introduced into cells by retroviral infection is more efficient than that of genes introduced into cells by DNA transfection. J Virol. 1984 May;50(2):417–424. doi: 10.1128/jvi.50.2.417-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Scolnick E. M., Evans L. H., Duesberg P. H. Biological activity of the spleen focus-forming virus is encoded by a molecularly cloned subgenomic fragment of spleen focus-forming virus DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1401–1405. doi: 10.1073/pnas.78.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Ong E. S., Rosenfeld M. G., Verma I. M., Evans R. M. Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science. 1984 Sep 7;225(4666):993–998. doi: 10.1126/science.6089340. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Stocking C., Johnson G. R., Kluge N., Kollek R., Franz T., Hess N. Transforming genes and target cells of murine spleen focus-forming viruses. Adv Cancer Res. 1987;48:193–355. doi: 10.1016/s0065-230x(08)60693-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K. Employment of a [3H]thymidine-incorporation assay to distinguish the effects of different Friend erythroleukemia-inducing retroviruses on erythroid cell proliferation. J Natl Cancer Inst. 1986 Jul;77(1):241–245. [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Kabat D. Plasma membrane glycoproteins encoded by cloned Rauscher and Friend spleen focus-forming viruses. J Virol. 1980 Sep;35(3):844–853. doi: 10.1128/jvi.35.3.844-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Tissue tropism of a leukemogenic murine retrovirus is determined by sequences outside of the long terminal repeats. Proc Natl Acad Sci U S A. 1986 May;83(10):3376–3380. doi: 10.1073/pnas.83.10.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Scolnick E., Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3' gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]