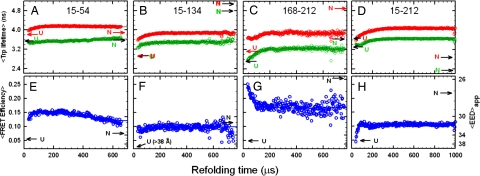
Fig. 3.
Average excited-state lifetimes of tryptophan as a function of refolding time following an 8 to 0.8 M urea concentration jump for four FRET pairs (green) and donor-only controls (red). (A) Pair 15-54. (B) Pair 15-134. (C) Pair 168-212. (D) Pair 15-212. (E–H) The corresponding FRET efficiencies are shown. The 〈EED〉app calculated from the Förster equation are indicated on the right vertical axis. The uncertainty in the average lifetimes, determined from controls in which no kinetics are present, is ≈0.015 ns. A consistent set of kinetics is observed in the average Trp lifetimes and the FRET efficiencies, suggesting that the observed kinetics are not an artifact of the mutations or acceptor chromophore labeling. Data were recorded at 21°C.