Table 3.
Challenging ARCM Substrates.
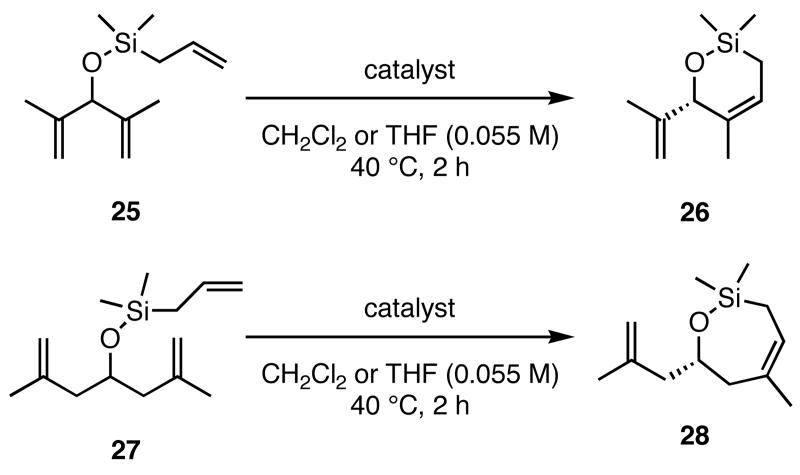 | ||||
|---|---|---|---|---|
| 25 to 26a | 27 to 28a | |||
| catalyst | ee b (%) | conv c (%) |
ee b (%) | conv c (%) |
| 2a | 16 | 72 | −5 | >98 |
| 3a | 45 | 92 | −5 | >98 |
| 5a | 30 | 93 | −8 | >98 |
| 2b | nd | <2 | 3 | 15 |
| 3b | nd | 6 | 15 | 18 |
Reactions with 2a-5a (2 mol %) run in CH2Cl2; catalysts 2b and 3b generated by stirring 2a and 3a (4 mol %) in THF with 25 equiv NaI.
Enantiomeric excesses determined by chiral GC; see supporting information for chromatograms and proof of absolute stereochemistry.
Determined by 1H NMR spectrum of crude reaction mixture. nd = not determined
