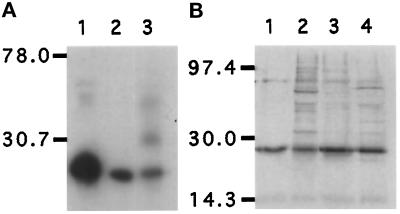
Figure 1.
Characterization of anti-GAIP antibodies demonstrating that they recognize only GAIP, a ∼25 kDa protein, by immunoblotting (A) or immunoprecipitation (B) of a lysate from AtT-20 pituitary cells stably expressing HA-GAIP. (A) Cells were homogenized, centrifuged at 600 × g, and the postnuclear supernatant was solubilized in Laemmli sample buffer. The proteins (50 μg/lane) were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with anti-HA (1 μg/ml; lane 1); anti-GAIP (C) antiserum (1:8000; lane 2), or anti-GAIP (N) antiserum (1:4000; lane 3) followed by appropriate goat anti-mouse or anti-rabbit IgG coupled to HRP and detection by ECL. (B) Cells were labeled for 4 h in 100 μCi/ml Easytag (Dupont, Boston, MA) after which they were homogenized, and a postnuclear supernatant was prepared and solubilized in RIPA buffer. The lysate was precleared with 20 μl Protein A/G-Sepharose (mAb 16B12) or protein A-Sepharose (rabbit sera), after which GAIP was precipitated with anti-HA (5 μg/ml; lane 1), anti-GAIP (N) (lane 2), anti-GAIP (C) (lane 3), or anti-GAIP23–217 (lane 4) antisera diluted 1:333, and the immunoprecipitates were separated by SDS-PAGE and detected by autoradiography.