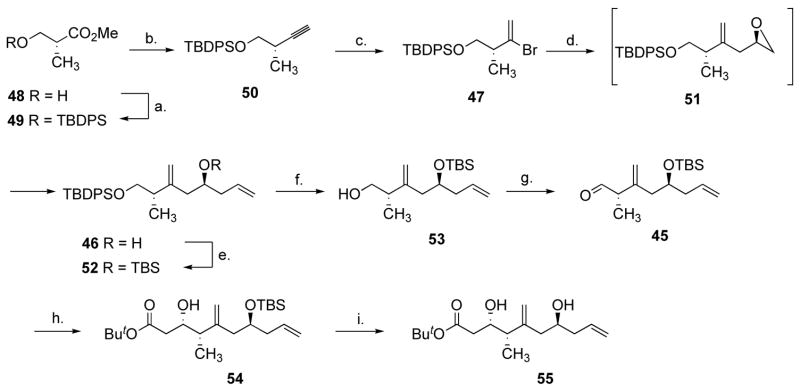
Scheme 11.
Synthesis of the alkene coupling partner.a
Reagents and conditions: (a) 1.0 equiv. TBDPSCl, 1.3 equiv. imidazole, CH2Cl2, 23 °C, 0.5 h. (b) 1.15 equiv. DIBAL-H, CH2Cl2, −78 °C, 60 min, then 1.35 equiv. MeOH, −78 °C to 24 °C then added to 2.5 equiv. CH3(CO)CHN2P(O)(OMe)2, 2.5 equiv. NaOMe, THF, −78 °C to 0 °C, 20 min, 83% (2 steps). (c) 2.0 equiv. 9-Br-9-BBN, CH2Cl2-hexane, 0 °C, 6 h then 14 equiv. AcOH, 0 °C, 1 h, 96%. (d) 2.0 equiv. t-BuLi, ether, −78 °C, 1 h, then 1.3 equiv. ThCu(CN)Li, THF, −78 °C to −45 °C, −45 °C, 1 h, then 2.0 equiv. 34, THF, −45 to 0 °C, 0 °C, 5 h, then 2.0 equiv. vinyllithium, 2.0 equiv. BF3.Et2O, THF, −78 °C, 20 min, 71%. (e) 1.8 equiv. TBSOTf, 4.0 equiv. 2,6-lutidine, CH2Cl2, 0 °C, 5 min. (f) 1.2 equiv. TBAF.3H2O, 1.2 equiv. AcOH, DMF, 23 °C, 13 h, 77% (2 steps). (g) 2.0 equiv. (COCl)2, 4.0 equiv. DMSO, 4.6 equiv. Et3N, CH2Cl2, −78 °C to −20 °C, 20 min. (h) 4.0 equiv. t-BuOAc, 4.0 equiv. LDA, THF-hexanes, −78 °C, 1 h, then 45, THF, −78 °C, 10 min, 78% (2 steps). (i) 1.5 equiv. TBAF, THF, 24 °C, 4 h, 89%.