Scheme 2.
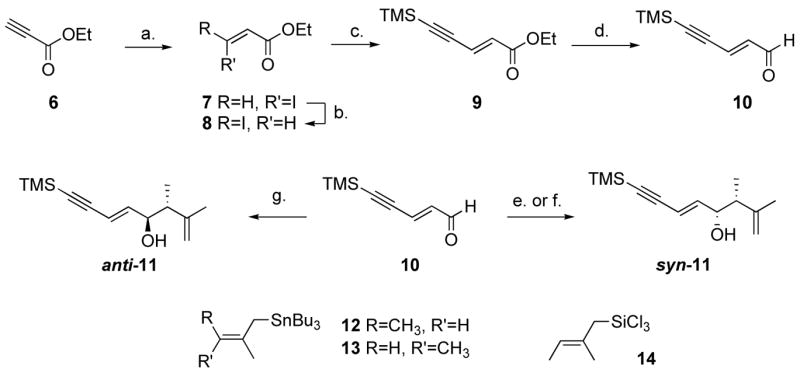
Synthesis of racemic enyne systems.a
a Reagents and conditions: (a) 1.5 equiv. NaI, AcOH, 70 °C, 13 h, Z/E>49:1. (b) 0.01 equiv. aq. HI, benzene, 1.7 M, 80 °C, 8 h, E/Z 16:1. (c) 1.1 equiv. trimethylsilylacetylene, 0.005 equiv. CuI, 0.01 equiv. Pd(PPh3)2Cl2, Et3N, 50 °C, 13 h, 81% (3 steps). (d) 1.1 equiv. DIBAL-H, toluene, −95 °C, 1 h, 70%. (e) 1.1 equiv. BF3.Et2O, 1.3 equiv. 12, CH2Cl2, −78 °C, 5 min, quant., syn/anti 2.6:1. (f) 1.1 equiv. BF3.Et2O, 1.3 equiv. 13, CH2Cl2, −78 °C, 5 min, quant., syn/anti 6.5:1. (g) 1.0 equiv. HMPA, 2.0 equiv. 14, CH2Cl2, −78 °C, 12 h, 23%, syn/anti 1:19.
