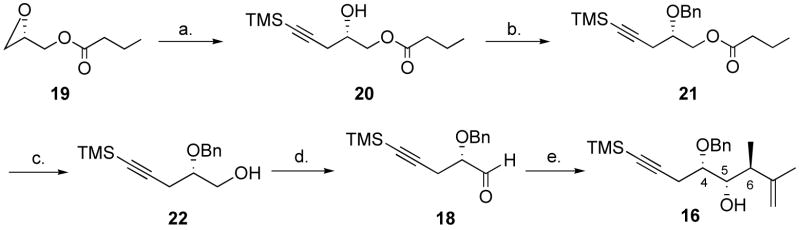
Scheme 4.
Synthesis of alcohol 16.a
a Reagents and conditions: (a) added to 1.3 equiv. lithium acetylide, 1.3 equiv. AlMe3, then 1.3 equiv. BF3.Et2O added, ether, −78 °C, 0.5 h. (b) 2.0 equiv. benzyl-2,2,2-trichloroacetimidate, 0.2 equiv. TfOH, dioxane, 24 °C, 0.5 h. (c) 1.3 equiv. DIBAL-H, CH2Cl2, −78 °C, 15 min. (d) 2.0 equiv. oxalyl chloride, 4.0 equiv. DMSO, 5.0 equiv. Et3N, CH2Cl2, −78 °C to 0 °C, 71% (4 steps). (e) 1.0 equiv. SnCl4, 2.0 equiv. 17, CH2Cl2-pentane 1:1, −110 °C, 15 min, 77%, 9:1 d.r at C-6.