Table 3.
Addition of alkene 65a–b to enynes 64a–g.
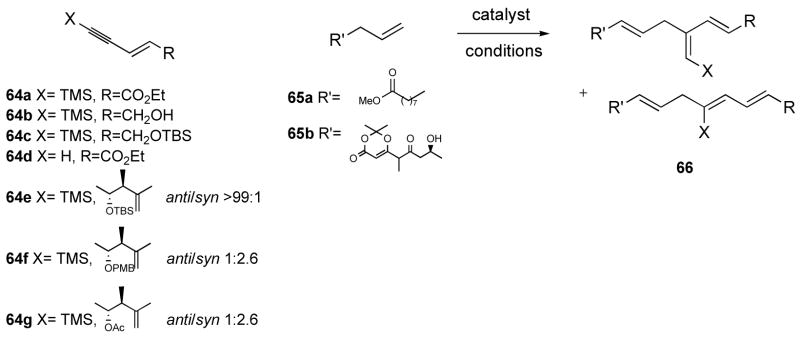 | |||||||
|---|---|---|---|---|---|---|---|
| entrya | alkyne | alkene | catalyst (mol%) | solvent | temp. °C | product % (brsm)b | branched to linear ratio |
| 1 | 64a | 65a | 67 (10) | acetone | 24 | 66aa 31 (70) | 1:0 |
| 2 | 64a | 65a | 67 (10) | DMF | 24 | 66aa 5 (73) | n.d. |
| 3 | 64a | 65a | 67 (10) | DMF | 55 | 66aa 5 (65) | n.d. |
| 4 | 64b | 65a | 67 (10) | acetone | 24 | no reaction | - |
| 5 | 64c | 65a | 67 (10) | acetone | 24 | 66ca 45 (75) | 1:0 |
| 6 | 64d | 65a | 67 (10) | acetone | 24 | 66da 26 (75) | 1:2 |
| 7 | 64d | 65a | 67 (10) | DMF | 24 | 66da 36 (78) | 1.8:1 |
| 8 | 64d | 65a | 67 (10) | DMF | 55 | 66da 50 (76) | 1.8:1 |
| 9 | 64d | 65a | 67 (10) | DMF | 70 | 66da 56 (68) | 2.7:1 |
| 10 | 64d | 65a | 68 (5) | MeOH | 65 | 66da 13 (53) | 2.3:1 |
| 11 | 64e | 65a | 67 (10) | acetone | 24 | 66ea 46 (65) | 1:0 |
| 12 | 64f | 65b | 67 (10) | acetone | 24 | 66fb 27 (100) | n.d. |
| 13 | 64f | 65b | 67 (100) | acetone | 24 | 66fb 10 (25) | n.d. |
| 14 | 64f | 65b | 67 (10) | DMF | 60 | 66fb no reaction | - |
| 15 | 64g | 65b | 67 (10) | acetone | 24 | 66gb 10 (40) | n.d. |
| 16c | 64g | 65b | 67 (10) | acetone | 24 | 66gb traces | - |
| 17 | 64g | 65b | 67 (10) | DMF | 65 | 66gb no reaction | - |
All reactions were run at 0.1M for 1–4 h using a 1:1 ratio of alkene to alkyne.
brsm indicates the yield based on recovered alkene.
1 equivalent of malonic acid was added.
