Table 2.
Reactions with Different Nucleophilic Moieties.a
| substrate | product | time | yield | ee | |
|---|---|---|---|---|---|
| 1 | 2a (R=Ph) | (S)-1a | 1/4 h | 93% | 92% |
| 2 | 3a (R=Ph) | (S)-1a | 1 h | 89% | 91% |
| 3 | 2b (R=p-MeOPh) | 1b | 1/4 h | 94% | 92% |
| 4 | 3b (R=p-MeOPh) | 1b | 2 h | 86% | 92% |
| 5 | 2c (R=2-Naphthyl) | 1c | 1/4 h | 92% | 85% |
| 6 | 3c (R=2-Naphthyl) | 1c | 1/2 h | 94% | 85% |
| 7 | 2d (R=o-NO2Ph) | 1d | 12 h | 69% | 79% |
| 8 | 3d (R=o-NO2Ph) | 1d | 12 h | 69% | 72% |
| 9 | 2e (R=2-furyl) | 1e | 1/2 h | 81% | 93% |
| 10 | 2f (R=1-cyclohexenyl) | 1f | 7 h | 93% | 98% |
| 11 | 2g (R=2-methyl-1-propenyl) | 1g | 5 h | 89% | 98% |
| 12 | 2i (R=PhC C) | 1i | 1/4 h | 76% | 89% |
| 13 | 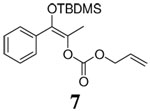 |
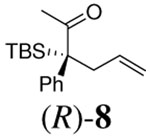 |
12 h | 94% | 80% |
| 14 | 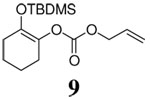 |
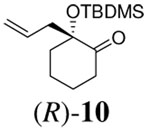 |
10 h | 96% | 64% |
| 15 |  |
4i | 2 h | 95% | 99% |
All reactions were performed on a 0.2 mmol scale at 0.1 M in dioxane at 23 °C using 2.5 mol% Pd2(dba)3CHCl3 and 5.5 mol% ligand L; the yields were isolated yields and ee values were determined by chiral HPLC.
