Abstract
Background and Aims
Spring ephemerals have a specific life-history trait, i.e. shoot growth and sexual reproduction occur simultaneously during a short period from snowmelt to canopy closure in deciduous forests. The aim of this study is to clarify how spring ephemerals invest resources for seed production within a restricted period.
Methods
In order to evaluate the cost of reproduction of a typical spring ephemeral species, Adonis ramosa, an experiment was conducted comprising defoliation treatments (intact, one-third and two-thirds leaf-cutting) and fruit manipulations (control, shading and removal) over two growing seasons. In addition, measurements were made of the movements of carbon assimilated via 13C tracing.
Key Results
Survival rate was high irrespective of treatments and manipulations. The proportion of flowering plants and plant size decreased as a result of the defoliation treatments over 2 years, but the fruit manipulations did not affect flowering activity or plant size. Seed set and seed number decreased as a result of fruit shading treatment, but the defoliation treatments did not affect current seed production. Individual seed weight also decreased in the second year due to fruit shading. The 13C tracing experiment revealed that young fruits had photosynthetic ability and current photosynthetic products from leaves were mainly transferred to the below-ground parts, while translocation to fruit was very small even when fruit photosynthesis was restricted by the shading treatment.
Conclusions
Current foliage photosynthetic products are largely stored in the below-ground parts for survival and future growth, and about one-third of the resources for seed production may be attained by fruit photosynthesis. Therefore, the trade-off between current seed production and subsequent growth is weak. The cost of seed production may be buffered by sufficient storage in the below-ground organs, effective photosynthesis under high irradiation and self-assimilation ability of fruits.
Key words: Adonis ramosa, carbon transfer, cost of reproduction, defoliation, non-foliar photosynthesis, resource allocation, seed production, spring ephemeral
INTRODUCTION
The cost of reproduction is expressed as trade-offs between present reproduction and future survival, growth and reproduction (Reznick, 1985; Stearns, 1992). Many studies have detected evidence of the cost for plant reproduction, but an almost equal number of studies have reported no evidence of trade-offs between current reproduction and future reproduction and/or performance (reviewed in Obeso, 2002). The lack of cost detection is usually because of: (1) potentially low or restricted resource investment in reproduction (Jennersten, 1991; Hemborg and Karlsson, 1998); (2) possession of a large resource budget relative to the expense for single reproduction (Primack and Hall, 1990; Ehrlén and van Groenendael, 2001); (3) formation of physiologically integrated modular structures (Sprugel et al., 1991; Obeso, 1998); (4) compensative acceleration of photosynthetic abilities during reproduction (Herold, 1980; Lehtilä and Syrjänen, 1995); and/or (5) photosynthetic function of reproductive organs (Bazzaz et al., 1979; Jurik, 1985; Antlfinger and Wendel, 1997; Guido and Hardy, 2003). Decrease in the cost of reproduction by these mechanisms may make it difficult to detect the trade-offs between current reproduction and future reproduction or performance with short-term experiments on reproductive output and/or resource budget manipulations (Primack and Hall, 1990; Primack and Stacy, 1998; Ehrlén and van Groenendael, 2001).
The effects of flower removal, hand-pollination, shading and defoliation on current and future reproduction have been shown to vary greatly depending on species or timing of manipulations in previous studies (reviewed in Obeso, 2002). This is because the resource budget for current reproduction differs among species and/or changes seasonally even in a single species. In comparisons of defoliation effects on reproductive output (Obeso, 1993), most woody species tended to decrease seed production, but 44 % of herbaceous species did not change seed production after defoliation. However, some woody species did not use current photosynthetic products by the leaves but used storage resources in reproductive stems (Obeso and Grubb, 1993; Miyazaki et al., 2002). In some plants, current photosynthetic products early in the season (flowering stage) are used for seed production, whereas those late in the season (fruiting stage) are used for storage, i.e. for growth and flower production in the next season (Marquis, 1992; Garcia and Ehrlén, 2002). Therefore, clarification of source–sink relationships based on the life cycle of individual species is very important to understand the cost of reproduction (Obeso and Grubb, 1994).
Spring ephemerals growing under deciduous forests have characteristic life cycles, with emerging and blooming in early spring, setting fruits within a short period, and with above-ground parts commonly dying back before or soon after canopy closure (Muller, 1978; Schemske et al., 1978; Kawano, 1985; Motten, 1986). They usually have high photosynthetic abilities and can assimilate effectively during a short photosynthetic season (Taylor and Pearcy, 1976). Because the reproductive period and whole growing season of spring ephemerals are largely overlapping, allocation patterns of photosynthetic products between current reproduction and storage for next season should be a crucial life-history strategy (Lubbers and Lechowicz, 1989; McKenna and Houle, 2000). For example, Lubbers and Lechowicz (1989) detected that there is a trade-off between storage and current reproduction in a perennial spring ephemeral, Trillium grandiflorum, in which resource allocation to current reproduction has priority over survival and future reproduction. They speculated that this might reflect the selective effects of unpredictable pollination success given that seed production of T. grandiflorum was pollen-limited. However, because storage function and pollination situation vary greatly among spring ephemerals (Kawano, 1985; Motten, 1986; Kudo et al., 2004), trade-off relationships between current reproduction and future performance should vary depending on the life-history traits of individual species.
In the present study, the cost of reproduction and the origin of resources for seed production were surveyed in a typical spring ephemeral, Adonis ramosa, growing under deciduous forests in northern Japan. Despite very early flowering under cool conditions (early April in the test sites), this species shows a stable high fruit production from year to year (Kudo et al., 2004). The aim was to clarify how this species gains and invests resources for seed production within a short growing season. Because flowering occurs prior to leaf emergence, flower production must be supported by storage resources. Leaf deployment is completed late in the flowering season, and above-ground parts die back by canopy closure at the time of seed dispersal. The question of interest was where do the resources for seed production come from? There are three possible carbon sources for seed production: below-ground storage (roots and rhizomes), current foliage photosynthetic products, and photosynthetic products from the plant's own fruits. An experiment was conducted comprising of defoliation and fruit manipulations (control, shading and removal) to clarify the major source for reproduction. (1) If storage resources are a major source, a reduction of current photosynthetic products via defoliation should decrease flower and fruit production in the subsequent year. By contrast, flower removal may increase growth and/or reproductive activity in the subsequent year, if fruit production is costly. (2) If current foliage assimilation supports fruit production, defoliation should decrease the seed production in the current year. In addition, flower removal may increase the growth and/or reproductive activity in the subsequent year because extra resources are allocated to storage organs without fruits. (3) If fruit development is supported by its own assimilation, fruit shading should decrease seed production. Furthermore, flower removal may not influence subsequent growth and/or reproductive activity because the cost of seed production is self-supported. In addition to these experimental approaches, carbon movement within plants was directly traced using a stable isotope of carbon, 13C.
MATERIAL AND METHODS
Plant material
Adonis ramosa (Ranunculaceae) is one of the earliest blooming spring ephemerals growing under deciduous forests of central to northern Japan. Flowering in Hokkaido usually occurs within 1–3 d after snowmelt around early to mid-April. Usually, only one flower, which is gold-coloured and parabolic in shape, is produced per stem. Leaf emergence starts during the flowering season, fruits mature during late May to early June, and above-ground parts senesce by mid-June. Thus, the vegetative growth and reproductive seasons greatly overlap. Seed set of A. ramosa is consistently high over years, as determined previously (Kudo et al., 2004), probably because of self-compatibility and effective pollinator attraction by heliotropic flower movements (Kudo, 1995).
Research site
This study was mainly conducted in a deciduous forest of the Tomakomai Experimental Forest of Hokkaido University (TOEF; 42°40′N, 141°36′E) in Hokkaido, northern Japan. Mean monthly temperature ranges from –3·2 to 19·1 °C, and annual precipitation is 1200 mm. Snow usually covers the ground from early December to early April. Leaf emergence of the canopy trees starts in mid-May and canopy closure is completed by mid-June. The 13C tracing experiment was conducted in another deciduous forest in Ebetsu (43°25′N, 141°32′E) in which timing of snowmelt and flowering phenology are similar to those at TOEF.
Defoliation and fruit manipulation experiment
In spring 2003, soon after snowmelt, 135 plants with flowering buds were arbitrarily selected and tagged with numbered sticks within a population at TOEF. These plants were divided into nine groups of 15 plants. Then, combinations of three defoliation treatments (intact, one-third leaf-cutting, two-thirds leaf-cutting) and three fruit manipulations (control, removal, shading) were allocated, i.e. 3 × 3 treatments. Leaf cutting involved the removal of one- or two-thirds in area of the distal part for all leaves within a plant soon after leaf expansion during the flowering season. Fruit removal was accomplished by removal of flowers during the flowering season; hence, the cost of fruit and seed production was removed. The fruit shading treatment involved bagging of young fruits soon after flowering with waterproof paper bags that cut off > 99 % of the light, by which photosynthetic assimilation by fruits was inhibited. The same treatments were repeated in 2004 for the same individuals. Survival and flowering conditions of individual plants were monitored in 2004 and 2005.
Basal stem diameter of shoots at flowering stage (D, mm) was used as a representative measure of plant size because there is a significant correlation between total plant mass [g (d. wt), W] and stem diameter in this species (ln W = 0·804 × ln D2 – 0·649, r = 0·71, P = 0·0005, n = 20; our unpublished data). Stem diameter at flowering season was measured in 2003, 2004 and 2005. Effects of the treatments on plant growth were assessed by comparing the changes in stem diameter between 2003 and 2004 or 2005. Seed-set success (proportion of ovules setting seeds), seed number and individual seed weight (d. wt) per fruit were measured in 2003 and 2004 to evaluate the effects of the treatments on current reproductive output.
13C tracing experiment
Two 13C tracing experiments were conducted in May 2003. In the first, the assimilative ability of young fruits was assessed via 13CO2 exposure to fruits under natural light and shaded conditions. Early in the fruiting season, 20 plants with young fruits were selected, and they were divided into light-intact (eight plants) and shading groups (12 plants). A fruit was enclosed in a transparent plastic bag with two Eppendorf tubes, which contained 0·1 g Ba13CO3 (13C = 99·7 at.%; Syoko-Tsusyou Inc., Tokyo, Japan) and two plastic tubes filled with 5 mL lactic acid, and sealed with adhesive tape. 13CO2 was generated by reacting BaCO3 with lactic acid within the bag. CO2 exposure was conducted for 2 d, from 0600 to 1700 h of the next day, and the CO2 generation was carried out twice, at 0600 h on each day. After 35 h of exposure, the above-ground part was removed, and fruit, stem and leaves were sampled separately. For below-ground parts (rhizome and roots), sampling was only from three plants in each group to conserve the population. These samples were immediately brought to the laboratory in an insulated box filled with ice, and kept in a freezer at below –30 °C. They were freeze-dried (Flexi-Dry FD-1-54A; FTS Systems Inc., Stone Ridge, NY, USA) and ground to a fine homogeneous powder in the laboratory using liquid nitrogen and mortar.
In the second experiment, assessment was made to determine whether carbon assimilation by leaves supports fruit development under light-intact and fruit-shading conditions. The experimental design was identical to the first experiment but 13CO2 exposure was performed for leaves, and 12 plants for the light-intact and eight plants for the fruit-shading group were used.
To assess the initial amount of 13C in the plant parts, ten plants from the same population were sampled but these were at least 5 m apart from the 13CO2 exposed plants as unlabelled controls. They were cut off and the above-ground parts were treated in the same way as for exposed samples.
The abundance of 13C was analysed using a Finnigan MAT 252 isotope ratio mass spectrometer (Finnigan MAT GmbH, Bremen, Germany).
Statistical analyses
Survival of plants in the second (2004) and third (2005) year was analysed by a logistic regression, separately, in which fruit manipulation (control, shading and removal), defoliation (intact, one-third and two-thirds cutting), stem diameter in the previous year (to evaluate the size effect) and seed production in the previous year (to evaluate reproductive cost) were dependent variables. Flowering occurrence in the second (2004) and third (2005) year was also analysed by a logistic regression of the same model.
Changes in stem diameter after 1 year (ΔD03–04) and 2 years (ΔD03–05), as a measure of plant growth, were compared by two-way ANOVA in which the effects of fruit manipulation and defoliation were considered, followed by the Tukey–Kramer post-hoc test.
For the analyses of seed set and seed number per plant, the generalized linear model (GLM) was used for each year (2003 and 2004) and considered a logit function and a binomial error distribution for seed set, and a logarithmic link function and a Poisson error distribution for seed number, as data distributions obviously deviated from a normal distribution even after any data transformation. In GLMs, fruit manipulation (control and shading), defoliation (intact, one-third and two-thirds cutting) and stem diameter in the current year (i.e. plant size) were considered as independent variables. For the defoliation treatments, value of 1·0 was assigned for intact, 0·67 for one-third cutting and 0·33 for two-thirds cutting for GLM analyses.
Mean seed weight per fruit was compared by repeated-measures two-way ANOVA in which fruit manipulation and defoliation were considered as independent variables and year (2003 and 2004) was a repeated variable.
The relative abundance of 13C in plant organs (fruit, stem, leaf, below-ground parts) was indicated by the index at.%. At.% of 13C was calculated as follows (Hasegawa et al., 2003): At.% = amount of 13C/(amount of 12C + amount of 13C) × 100. The carbon increment, Cinc, in each organ was calculated as Cinc = (Alabel–Acont)/100 × W × Cperc, where Alabel is 13C at.% of the focal organ of 13C-labelled samples, Acont is 13C at.% of the unlabelled control, W is the dry weight of the focal organ and Cperc is carbon concentration. In some cases, acquired Cinc showed negative values because of measurement errors of the mass spectrometer. When Cinc was less than zero, it was assumed to be zero. The difference of transported carbon between the light-intact and fruit-shading group was analysed by Mann–Whitney U-test for each organ.
RESULTS
Shoot survival and flowering activity
Most plants survived to the second (87–100 %) and third (73–100 %) year of the experiment (Table 1). Results of logistic regression revealed that none of the fruit manipulations, defoliations, plant size or seed production in the previous year influenced survival (P > 0·10). The proportion of flowering plants was 60–93 % in 2004, but decreased to 20–73 % in 2005 (Table 1). The number of control flowering plants (n = 15) was 14 in 2004 and nine in 2005. Although no significant trend in flowering activity was detected in 2004 (P > 0·05, logistic regression), flowering occurrence in 2005 was negatively influenced by the intensive defoliation (two-thirds cutting) and positively influenced by diameter size in the previous year (P = 0·018 and 0·0076, respectively). Fruit manipulation and seed production in the previous year did not influence flowering occurrence (P > 0·10). Mean (± s.d.) stem diameter in 2005 was 4·31 ± 0·69 mm (n = 62) in flowering plants and 3·25 ± 0·69 mm (n = 55) in non-flowering plants.
Table 1.
Frequencies of shoot occurrence (survival) and flowering in the second (2004) and third (2005) years of the fruit manupulation (control, fruit shading and fruit removal) and defoliation (intact, one-third leaf-cutting and two-thirds leaf-cutting) experiments
| Survival (%) |
Flowering (%) |
|||||
|---|---|---|---|---|---|---|
| Fruit manipulation | Defoliation | n | 2004* | 2005* | 2004* | 2005† |
| Control | Intact | 15 | 100 | 100 | 93 | 60 |
| 1/3-cut | 15 | 93 | 80 | 80 | 53 | |
| 2/3-cut | 15 | 87 | 73 | 80 | 40 | |
| Shading | Intact | 15 | 100 | 100 | 73 | 73 |
| 1/3-cut | 15 | 100 | 87 | 60 | 33 | |
| 2/3-cut | 15 | 100 | 100 | 60 | 20 | |
| Removal | Intact | 15‡ | 93 | 92 | 80 | 54 |
| 1/3-cut | 15‡ | 100 | 92 | 73 | 54 | |
| 2/3-cut | 15 | 100 | 87 | 87 | 40 | |
* There are no significant differences in survival (both years) and flowering occurrence in 2004 by logistic regression.
† Flowering occurrence in 2005 is negatively correlated to defoliation (P = 0·053) and positively correlated to stem diameter in the previous year (P = 0·0051) by logistic regression.
‡ n = 13 in 2005 because tags of two plants were missing.
Vegetative growth
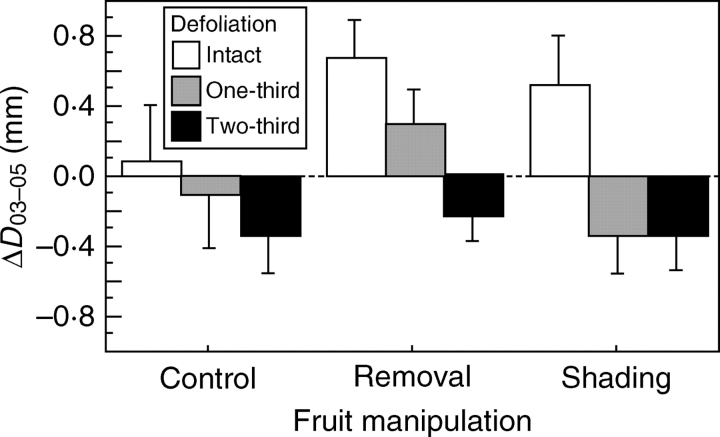
There was no significant difference in the changes in basal stem diameter from 2003 to 2004 (ΔD03–04) both in the fruit manipulation (F2,119 = 0·665, P = 0·52, ANOVA) and the defoliation treatment (F2,119 = 0·751, P = 0·47). By the second year, however, the changes in basal stem diameter (ΔD03–05) were significantly different among the defoliation treatments (F2,108 = 7·27, P = 0·0011); a significant difference was detected between intact and two-thirds cutting (P < 0·05, Tukey–Kramer post-hoc test), while the effect of the fruit manipulation was not significant (F2,108 = 1·79, P = 0·17; Fig. 1). These results indicate that intensive defoliation decreases plant growth at least after a 1-year interval but that seed production does not influence subsequent growth.
Fig. 1.
Changes in stem diameter in the fruit manipulation and defoliation experiments from 2003 to 2005 (ΔD03–05). Values are means ± s.e.
Seed production
Seed set of plants without fruit shading was 67 ± 21 % (s.d.) in 2003 and 60 ± 28 % in 2004, while values with fruit shading were 52 ± 24 % in 2003 and 41 ± 36 % in 2004 (Fig. 2A). GLM results revealed that seed set in 2003 decreased with fruit shading treatment and increased with plant size (stem diameter), but was not influenced by defoliation treatment (Table 2). Similar results were obtained also in 2004, but defoliation was positively related to seed set in that year (Table 2). This might have been caused by an external accident: fruits of two intact plants were eaten by rodents before maturation and this resulted in low seed production in the intact plants. When those damaged plants were removed from the analysis, no defoliation effect was detected for seed set.
Fig. 2.
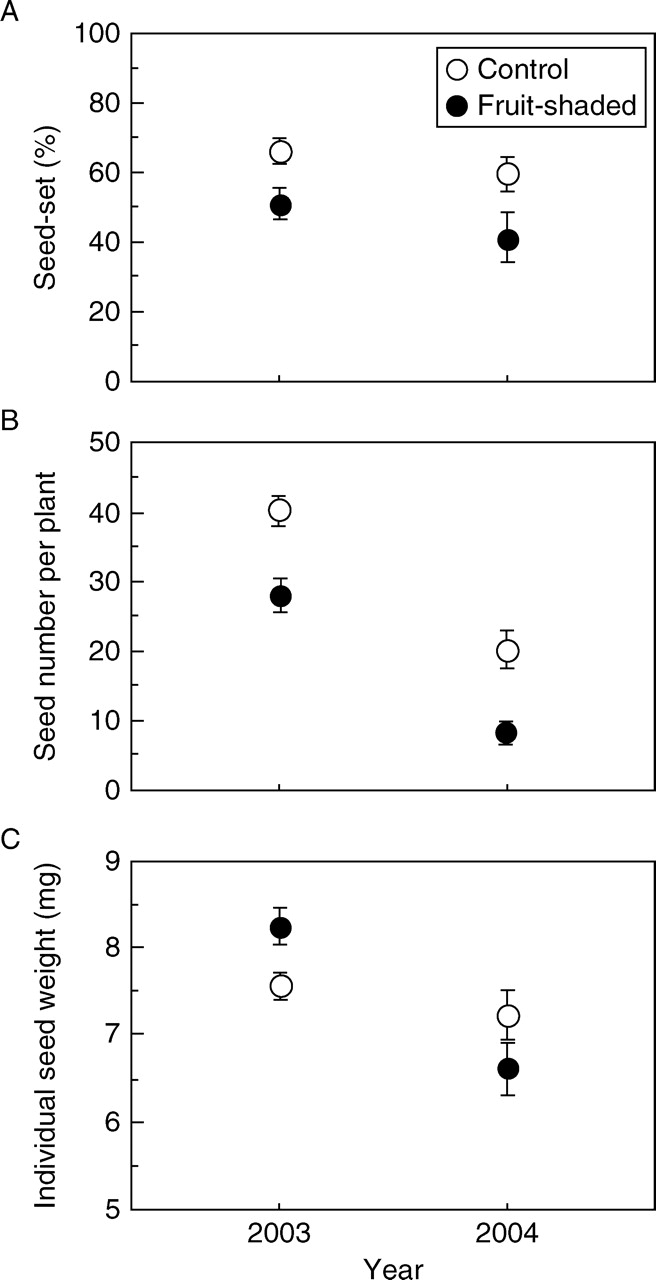
(A) Seed-set success, (B) seed number per plant and (C) individual seed weight in control and fruit-shaded plants in 2003 and 2004. Values are means ± s.e.
Table 2.
GLM results on seed set in 2003 (A) and 2004 (B). Intercept indicates control for fruit manipulation and no defoliation (intact)
| Coefficient | s.e. | Z-value | P-value | |
|---|---|---|---|---|
| (A) Seed set in 2003 | ||||
| Intercept | −0·228 | 0·191 | −1·197 | 0·231 |
| Shading | −0·527 | 0·059 | −8·819 | <0·0001 |
| Defoliation | −0·061 | 0·105 | −0·574 | 0·566 |
| Stem diameter | 0·238 | 0·043 | 5·538 | <0·0001 |
| (B) Seed set in 2004 | ||||
| Intercept | −0·382 | 0·269 | −1·419 | 0·156 |
| Shading | −0·850 | 0·086 | −9·845 | <0·0001 |
| Defoliation | −0·512 | 0·154 | −3·321 | 0·001 |
| Stem diameter | 0·283 | 0·064 | 4·398 | <0·0001 |
The pattern of seed production, i.e. number of seeds per plant, was almost the same as for seed set (Fig. 2B): seed production increased with plant size, decreased as a result of fruit shading, but was not influenced by defoliation effects in either year (Table 3). When only leaf-intact (no cutting) plants were compared, seed production was decreased by 23·7 % in 2003 and by 38·8 % in 2004 as a result of fruit shading.
Table 3.
GLM results on seed production in 2003 (A) and 2004 (B). Intercept indicates control for fruit manipulation and no defoliation (intact)
| Coefficient | s.e. | Z-value | P-value | |
|---|---|---|---|---|
| (A) Seed number in 2003 | ||||
| Intercept | 2·903 | 0·115 | 25·311 | <0·0001 |
| Shading | −0·253 | 0·039 | −6·575 | <0·0001 |
| Defoliation | −0·068 | 0·067 | −1·021 | 0·307 |
| Stem diameter | 0·199 | 0·025 | 7·917 | <0·0001 |
| (B) Seed number in 2004 | ||||
| Intercept | 1·856 | 0·192 | 9·642 | <0·0001 |
| Shading | −0·849 | 0·063 | −13·519 | <0·0001 |
| Defoliation | 0·025 | 0·106 | 0·234 | 0·851 |
| Stem diameter | 0·304 | 0·046 | 6·541 | <0·0001 |
Individual seed weight did not differ between control and fruit shading (F1,46 = 0·017, P = 0·89) nor among the defoliation treatments (F2,46 = 0·064, P = 0·94); however, it was lower in 2004 than in 2003 (F1,46 = 13·59, P = 0·0006) and a significant interaction was detected between year and fruit manipulation (F1,46 = 5·15, P = 0·028, repeated-measures two-way ANOVA). This is because the fruit-shading treatment decreased seed weight in the second year of the experiment (Fig. 2C).
13C tracing
Results of the 13C tracing experiment are shown in Table 4. When fruits were exposed to 13CO2, 0·669 mg 13C was assimilated in total under natural light conditions, 66 % of which remained in the fruits, 27 % in stems and < 5% in other organs. This means that young fruits can assimilate by themselves. By contrast, only 0·338 mg 13C was assimilated under fruit shading and more than 50 % of that remained in stems. Because the same amount of 13C in stems was detected in both light-intact and shading plants (0·179 mg), 13C in the stem seemed to be assimilated from its own photosynthesis. When fruits were exposed to 13CO2, some parts of the peduncles were also exposed. Thus, photosynthesis of the peduncle might cause high 13C concentration in stems. Under dark conditions, the amount of 13C assimilated by fruits decreased to 17 % of that under natural light conditions.
Table 4.
Amount of assimilated 13C in each organ in the 13C tracing experiment
| 13CO2 exposure | Organ | Light-intact | Shading | P-value* |
|---|---|---|---|---|
| Fruit | Fruit | 0·444 ± 0·231 (8) | 0·075 ± 0·051 (12) | 0·0002 |
| 66·4 % | 22·2 % | |||
| Leaf | 0·029 ± 0·009 (8) | 0·084 ± 0·089 (12) | >0·10 | |
| 4·3 % | 24·9 % | |||
| Stem | 0·179 ± 0·290 (8) | 0·179 ± 0·218 (11) | >0·10 | |
| 26·8 % | 52·9 % | |||
| Below-ground | 0·017 ± 0·017 (3) | 0·000 ± 0·000 (3) | – | |
| 2·5 % | 0·0 % | |||
| Leaf | Fruit | 0·097 ± 0·091 (11) | 0·066 ± 0·076 (8) | >0·10 |
| 0·8 % | 0·6 % | |||
| Leaf | 7·890 ± 1·850 (12) | 7·740 ± 3·680 (8) | >0·10 | |
| 65·5 % | 67·8 % | |||
| Stem | 1·090 ± 0·511 (12) | 1·260 ± 0·687 (8) | >0·10 | |
| 9·1 % | 11·0 % | |||
| Below-ground | 2·960 ± 0·910 (3) | 2·350 ± 1·810 (3) | – | |
| 24·6 % | 20·6 % |
13CO2 was exposed to young fruit and leaves under natural light conditions (light-intact) and fruit-shading conditions for 2 d. Mass of 13C (mg) was estimated by comparing 13C traced plants and control plants. Upper, mean ± s.d. (sample size); lower, percentage.
* Mann–Whitney U-test. A statistical test was not performed for below-ground parts due to the small sample size.
When leaves were exposed to 13CO2, 12·04 and 11·42 mg 13C were assimilated in total per plant in light-intact plants and fruit-shading plants, respectively. There was no significant difference in 13C distributions between the treatments (P > 0·10). About 66–68 % of 13C remained in the leaves, 21–25 % was transported to the below-ground parts, 9–11 % to stems and only 0·6–0·8 % to fruits. These results indicate that photosynthetic production by leaves is not used for current fruit production even when photosynthesis of fruits is restricted.
DISCUSSION
The present experiments have revealed that photosynthetic assimilation by fruits partly supported seed production of A. ramosa, and the contribution of current foliage assimilation to seeds was small. Current photosynthetic products are largely stored in the below-ground parts, which are used for future growth and flower production. Because extensive defoliation treatment (two-thirds cutting) over two seasons did not influence survival, this suggests that resources for several years may be stored in the below-ground parts. Because below-ground parts occupy about 85 % of total biomass at the flowering season (G. Kudo, unpubl. data), this species seems to have a typical polycarpic perennial life cycle of forest plants that shows a stable growth pattern as a result of the developed storage organs (Struik, 1965; Kawano, 1985).
Repeated defoliation (two-thirds cutting) significantly reduced flowering activity and plant size. Initial estimated biomass (2003) and after the experiments (2005), based on the allometric relationship between stem diameter and total dry weight, was 1·92 and 2·27 g in control plants (18·2 % increase), and 1·89 and 1·64 g in the two-thirds cutting plants (13·2 % decrease), respectively. The decrease in current photosynthetic carbon gain over 2 years might result in the reduction of plant size below a threshold size for reproduction in some plants (Snow and Whigham, 1989; Schmid et al., 1995). Therefore, there is a 2-year time lag for the responses of growth and flowering activity to the restriction of current assimilation in this species. Such delayed responses of reproductive performance to a reduction of resource storage may be common in herbaceous plants with developed storage organs (Primack and Hall, 1990; Ehrlén and van Groenendael, 2001).
Seed production was influenced by plant size and fruit shading. The significant reduction in seed number per plant by fruit shading (23·7 % in 2003 and 38·8 % in 2004) indicates that about one-third of total resource investment in seed production is supported by fruit photosynthesis, and the remaining two-thirds is largely from the below-ground storage. Because neither seed production in the previous season nor flower-removal manipulation influenced subsequent plant size, the cost of seed production may be small in this species. However, individual seed weight significantly decreased after repeated fruit shading (Fig. 2C). This suggests that resource investment in seed production from the storage organ was decreased when fruit photosynthesis was continuously restricted over several years, probably because extra resources (originating from storage organs) were used for fruit development in the previous season. Thus, photosynthesis by fruits acts as a buffer for stable seed production in this species.
Continuous flower production from year to year may be costly in this species. Only 60 % of plants flowered in the third year of the experiment under natural conditions (Table 1), and seed production of control plants in 2004 decreased to half of that in 2003 (Fig. 2B). This is because ovule number per flower decreased as a result of repeated flowering, indicating a reduction of resource availability for flower production. There is a positive correlation between ovule number and total flower mass in this species (r = 0·78; our unpubl. data). Because total size of control plants increased throughout the experimental period as mentioned before, A. ramosa may have a conservative strategy in terms of survival and vegetative growth over sexual reproduction.
As already discussed, seed production of a perennial spring ephemeral herb, Trillium grandiforum, was supported by current photosynthetic products from the leaves, and resource allocation to current reproduction was achieved at the expense of allocation to storage (Lubbers and Lechowicz, 1989). By contrast, only a small amount of current photosynthetic products was used for current reproduction in A. ramosa even when photosynthesis by fruits was restricted. Furthermore, the compensative support for seed production by below-ground storage was insufficient when carbon assimilation by fruits was restricted, as suggested by the fact that fruit shading significantly decreased seed production. Nevertheless, A. ramosa shows consistently high seed production under natural conditions (Kudo et al., 2004). This consistently high seed-set ability may be accomplished by: (1) the heliotropic warming of flowers, by which plants can attract pollinators and warm reproductive organs under cool conditions (Kudo, 1995); (2) moderate selfing ability without pollinator visits (Kudo, 1995); (3) rapid reproduction under bright conditions before canopy closure; (4) developed storage function of below-ground parts; and (5) photosynthesis by fruits. It has been shown that the heliotropism of A. ramosa contributes significantly not only to pollinator attraction but also to fruit development after fertilization (Kudo, 1995). This means that the sun tracking behaviour of the flowers and young fruits accelerates photosynthetic activity of the reproductive organ, resulting in a decreasing cost of reproduction.
For spring ephemerals growing under high-light conditions before canopy closure, low temperature is the most limiting factor, which causes low pollinator activity, low metabolism and slow growth (Schemske et al., 1978; McKenna and Houle, 2000; Kudo et al., 2004). Adonis ramosa can attain consistently high reproductive output with small cost of reproduction due to the maximal use of the short bright season. However, life-history traits of spring ephemerals vary (e.g. Kawano, 1985; Motten, 1986), and different trade-off relationships between current reproduction and other life-history traits are expected (e.g. Lubbers and Lechowicz, 1989). This may cause various patterns of reproductive behaviour among co-existing spring ephemeral species.
ACKNOWLEDGEMENTS
We are grateful for the support of the staff of Tomakomai Experimental Forest, Hokkaido University, throughout this research, and to Dr M. Minagawa for providing facilities for stable isotope analysis. This research was partly supported by grants-in-aid for scientific research (Nos. 1537006 and 1637007) from the Ministry of Education, Science, Sport and Culture of Japan.
LITERATURE CITED
- Antlfinger AE, Wendel LF. Reproductive effort and floral photosynthesis in Spiranthes cernua (Orchidaceae) American Journal of Botany. 1997;84:769–780. [PubMed] [Google Scholar]
- Bazzaz FA, Carlson RW, Harper JL. Contribution to reproductive effort by photosynthesis of flowers and fruits. Nature. 1979;279:554–555. [Google Scholar]
- Ehrlén J, van Groenendael J. Storage and the delayed costs of reproduction in the understory perennial Lathyrus vernus. Journal of Ecology. 2001;89:237–246. [Google Scholar]
- Garcia MB, Ehrlén J. Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. American Journal of Botany. 2002;89:1295–1302. doi: 10.3732/ajb.89.8.1295. [DOI] [PubMed] [Google Scholar]
- Guido A, Hardy P. Non-foliar photosynthesis – a strategy of additional carbon acquisition. Flora. 2003;198:81–97. [Google Scholar]
- Hasegawa S, Koba K, Tayasu I, Takeda H, Haga H. Carbon autonomy of reproductive shoots of Siberian alder (Alnus hirsute var. sibirica) Journal of Plant Research. 2003;116:183–188. doi: 10.1007/s10265-003-0085-7. [DOI] [PubMed] [Google Scholar]
- Hemborg ÅM, Karlsson PS. Somatic costs of reproduction in eight subarctic plant species. Oikos. 1998;82:149–157. [Google Scholar]
- Herold A. Regulation of photosynthesis by sink activity – the missing link. New Phytologist. 1980;86:131–144. [Google Scholar]
- Jennersten O. Cost of reproduction in Viscaria vulgaris (Caryophyllaceae): a field experiment. Oikos. 1991;61:197–204. [Google Scholar]
- Jurik TW. Differential costs of sexual and vegetative reproduction in wild strawberry populations. Oecologia. 1985;66:394–403. doi: 10.1007/BF00378305. [DOI] [PubMed] [Google Scholar]
- Kawano S. Life history characteristics of temperate woodland plants in Japan. In: White J, editor. The population structure of vegetation. Dordrecht: Junk Publishers; 1985. pp. 515–549. [Google Scholar]
- Kudo G. Ecological significance of flower heliotropism in the spring ephemeral Adonis ramosa (Ranunculaceae) Oikos. 1995;72:14–20. [Google Scholar]
- Kudo G, Nishikawa Y, Kasagi T, Kosuge S. Does seed production of spring ephemerals decrease when spring comes early? Ecological Research. 2004;19:255–259. [Google Scholar]
- Lehtilä K, Syrjänen K. Positive effects of pollination on subsequent size, reproduction and survival of Primula veris. Ecology. 1995;76:1061–1072. [Google Scholar]
- Lubbers AE, Lechowicz MJ. Effects of leaf removal on reproduction vs. below-ground storage in Trillium grandiflorum. Ecology. 1989;70:85–96. [Google Scholar]
- Marquis RJ. A bite is a bite is a bite? Constrains on response to folivory in Piper arieianum (Piperaceae) Ecology. 1992;73:143–152. [Google Scholar]
- McKenna MF, Houle G. Why are annual plants rarely spring ephemerals? New Phytologist. 2000;148:295–302. [Google Scholar]
- Miyazaki Y, Hiura T, Kato E, Funada R. Allocation of resources to reproduction in Styrax obassia in a masting year. Annals of Botany. 2002;89:767–772. doi: 10.1093/aob/mcf107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motten AF. Pollination ecology of the spring wild-flower community of a temperate deciduous forest. Ecological Monographs. 1986;56:21–42. [Google Scholar]
- Muller RN. The phenology, growth, and ecosystem dynamics of Erythronium americanum in the northern hardwood forest. Ecological Monographs. 1978;48:1–20. [Google Scholar]
- Obeso JR. Does defoliation affect reproductive output in herbaceous perennials and woody plants in different ways? Functional Ecology. 1993;7:150–155. [Google Scholar]
- Obeso JR. Effects of defoliation and girdling on fruit production in Ilex aquifolium. Functional Ecology. 1998;12:486–491. [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Obeso JR, Grubb PJ. Fruit maturation in the shrub Ligustrum vulgare (Oleaceae): lack of defoliation effects. Oikos. 1993;68:309–316. [Google Scholar]
- Obeso JR, Grubb PJ. Interactive effects of extent and timing of defoliation and nutrient supply on reproduction in a chemically protected annual Senecio vulgaris. Oikos. 1994;71:506–514. [Google Scholar]
- Primack RB, Hall P. Costs of reproduction in the pink lady's slipper orchid: a four-year experimental study. American Naturalist. 1990;136:638–656. [Google Scholar]
- Primack RB, Stacy EA. Cost of reproduction in the pink lady's slipper orchid (Cypripedium acaule, Orchidaceae): an eleven-year experimental study of three populations. American Journal of Botany. 1998;85:1672–1679. [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Schmid B, Bazzaz FA, Weiner J. Size dependency of sexual reproduction and of clonal growth in two perennial plants. Canadian Journal of Botany. 1995;73:1831–1837. [Google Scholar]
- Snow AA, Whigham DF. Cost of flower and fruit production in Tipularia discolor (Orchidaceae) Ecology. 1989;70:1286–1293. [Google Scholar]
- Sprugel DG, Hinckley TM, Schaap W. The theory and practice of branch autonomy. Annual Review of Ecology and Systematics. 1991;22:309–334. [Google Scholar]
- Schemske DW, Willson MF, Melampy MN, Miller LJ, Verner L, et al. Flowering ecology of some spring woodland herbs. Ecology. 1978;59:351–366. [Google Scholar]
- Stearns SC. The evolution of life histories. New York: Oxford University Press; 1992. [Google Scholar]
- Struik GK. Growth patterns of some native annual and perennial herbs in southern Wisconsin. Ecology. 1965;46:401–420. [Google Scholar]
- Taylor RJ, Pearcy RW. Seasonal patterns of the CO2 exchange characteristics of understory plants from a deciduous forest. Canadian Journal of Botany. 1976;54:1094–1103. [Google Scholar]