Table 2.
Screening of solvents and basesa
 | ||||
|---|---|---|---|---|
| entry | base | solvent | T (°C) | yield (%)b |
| 1 | K2CO3 | n-butanol | 117 | 75 |
| 2 | K2CO3 | water | 100 | 35 |
| 3 | K2CO3 | ethylene glycol | 130 | 40 |
| 4 | K2CO3 | 2-ethoxyethanol | 130 | 76 |
| 5 | K2CO3 | diethylene glycol | 130 | 90 |
| 6 | Cs2CO3 | diethylene glycol | 130 | 5 |
| 7 | Na2CO3 | diethylene glycol | 130 | 82 |
| 8 | tert-BuOK | diethylene glycol | 130 | 11 |
| 9 | K3PO4 | diethylene glycol | 130 | 70 |
| 10 | NaOAc | diethylene glycol | 130 | 66 |
| 11 | TMP | diethylene glycol | 130 | 30 |
| 12 | Na2CO3 | 2-ethoxyethanol | 130 | 92d |
| 13 | K2CO3 | 2-ethoxyethanol | 130 | 75c |
| 14 | K2CO3 | 2-ethoxyethanol | 130 | 83c,d |
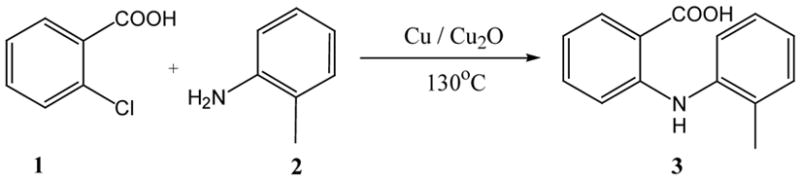
Reaction conditions: 2-Chlorobenzoic acid (1.38 g, 8.83 mmol), 1.05 equiv. of 2-methylaniline, 9 mol% of Cu, 4 mol% of Cu2O, 2.0 equiv. of base, 3 mL of solvents, 130°C for 24 hours.
Isolated yields.
Aniline was used.
1.0 equiv. of base was used.
