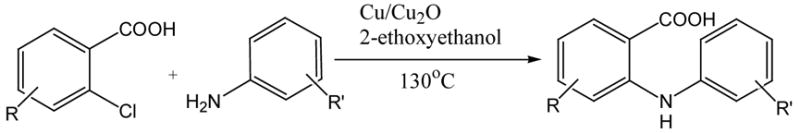
Table 3.
Arylation of aryl chlorides with aryl aminesa
 | ||||
|---|---|---|---|---|
| entry | aryl chloride | amine | product | Yield (%)b |
| 1 |
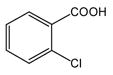
1 |

4 |
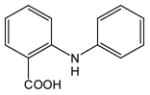
5 |
83 |
| 2 |

1 |
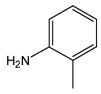
2 |

3 |
92 c |
| 3 |

1 |

6 |
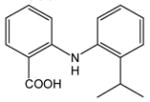
7 |
73 |
| 4 |

1 |

8 |

9 |
86 |
| 5 |

1 |
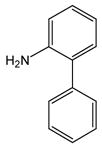
10 |

11 |
85 |
| 6 |

1 |

12 |

13 |
65 |
| 7 |

1 |

14 |

15 |
80 |
| 8 |

1 |

16 |

17 |
85 |
| 9 |

1 |
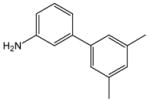
18 |

19 |
99 |
| 10 |

1 |
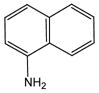
20 |

21 |
96 |
| 11 |

1 |

22 |

23 |
73 |
| 12 |

1 |

24 |

25 |
84 |
| 13 |

1 |
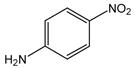
26 |

27 |
87 |
| 14 |

1 |

28 |

29 |
99 |
| 15 |

1 |
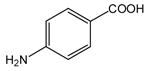
30 |

31 |
98d |
| 16 |

32 |
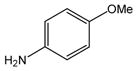
24 |
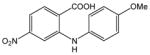
33 |
99 |
| 17 |
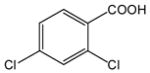
34 |

24 |

35 |
86 |
| 18 |

36 |
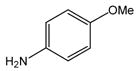
24 |
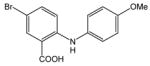
37 |
85 |
Reaction conditions: 2-Chlorobenzoic acid (1.38 g, 8.83 mmol), 1.05 equiv. of amine, 1.0 equiv. of K2CO3, 9 mol% of Cu, 4 mol% of Cu2O, 3 mL of 2-ethoxyethanol and 130°C for 24 hours.
Isolated yields.
Na2CO3 was used as base.
2.0 equiv. of base were used
