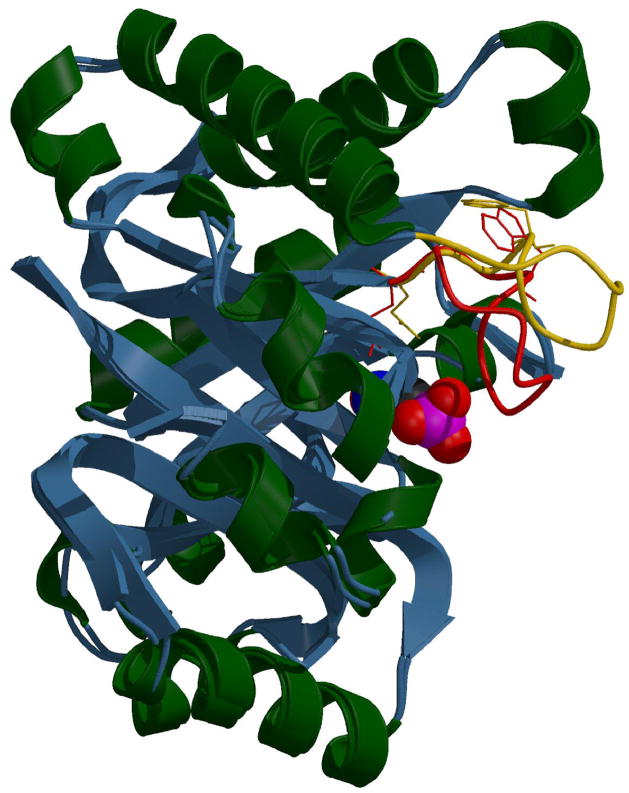
Figure 1.
Ribbon diagram of yeast triosephosphate isomerase with the overlaid conformations of catalysis controlling loop 6, yellow (open) and red (closed), shown as stick diagrams. The motion of the indole ring of Trp168 and the active site residue Glu165 shown as stick diagrams. The active site loop descends down to engulf the substrate and position some of the catalytic residues. The loop’s tryptophan rotates by about 50° between the two conformations