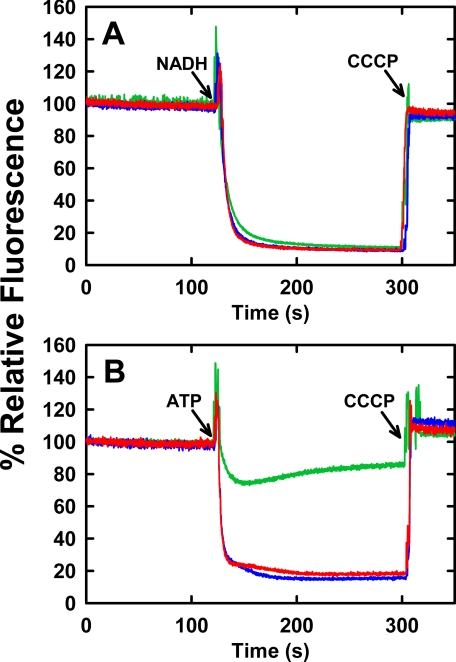
FIGURE 2.
NADH- or ATP-dependent proton electrochemical gradient formation by mutant F0F1 in membranes. 200 μg of membrane vesicle protein from strain DK8, harboring plasmid pBWU13 wild type or carrying the mutations for βE381C or βD380C, were suspended in 2 ml of buffer containing 10 mm HEPES-KOH, 300 mm KCl, 5 mm MgCl2, 1 μm valinomycin, and 1 μm acridine orange at pH 7.5, with vigorous mixing. Fluorescence intensity at 530 nm (excitation at 460 nm) was monitored at 25 °C. A, NADH-driven quenching. Proton pumping was initiated at the indicated time (arrow) by addition of 1 mm NADH. B, ATP-driven quenching. Proton pumping was initiated at the indicated time (arrow) by addition of 1 mm ATP. 1 μm carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added, as indicated (arrows) to abolish the proton gradient and establish the maximum fluorescence value. The fluorescence traces are depicted as follows: wild type (blue), βD380C (red), βE381C (green). The traces are representative of those from several trials.