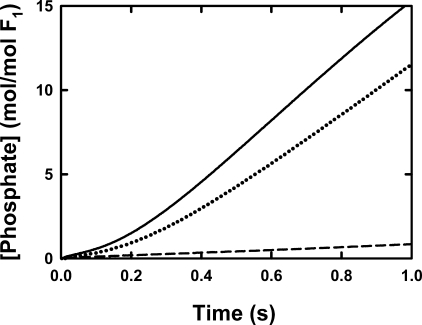
FIGURE 7.
Pre-steady release of Pi by βD380C-F1. Details are given under “Experimental Procedures” for the stopped-flow set up. F1 was prepared as described under “Experimental Procedures,” giving 4.0 ± 0.1 mol of nucleotide bound per mol of F1, equilibrated with 25 mm TES-KOH, 0.244 mm MgCl2, 0.2 mm EDTA, pH 7.5, at 25 °C, diluted to 1 μm with the same buffer, and loaded in syringe A. The ATP and MgCl2 concentrations in syringe B resulted in a final free Mg2+ of ∼50 and 105 μm Mg·ATP. The stopped-flow syringe also contained 20 μm MDCC-PBP. The traces of MDCC-PBP fluorescence show Pi released from the enzyme and were calibrated by measuring the fluorescence response to known concentrations of Pi. The response loses linearity above ∼16 μm because of saturation of Pi binding. Each trace is the average of at least three stopped-flow mixing experiments. The noise in the averaged data is within the thickness of the lines shown. The solid line shows the Pi release from the untreated F1; the dashed line depicts that from the γ-β cross-linked F1; and the dotted line is the Pi release from the partially reduced cross-linked sample after incubation with 5 mm DTT.