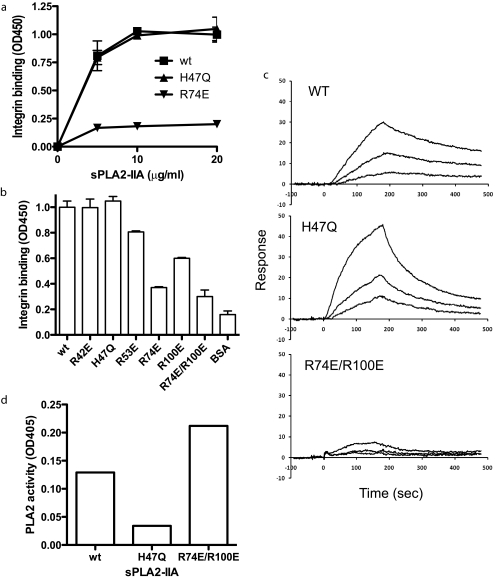
FIGURE 3.
Localization of the integrin-binding site of sPLA2-IIA. a, binding of sPLA2-IIA mutants to soluble αvβ3. Based on the docking model we introduced mutations in the integrin-binding interface of sPLA2-IIA. The mutant proteins were tested for binding to soluble integrin αvβ3. The low level background binding to BSA was subtracted. b, summary of the mutagenesis study of sPLA2-IIA-integrin interaction. The binding of soluble αvβ3 to immobilized Wt and mutant sPLA2-IIA was determined at the saturating conditions (20 μg/ml coating concentrations). The H47Q mutation is located in the catalytic center of the enzyme. c, the surface plasmon resonance study of sPLA2-IIA binding to αvβ3. Soluble integrin αvβ3 was immobilized to a sensor chip, and the binding of Wt sPLA2-IIA, and the H47Q and R74E/R100E mutants (concentrations at 2, 1, and 0.5 nm) was analyzed. KD was calculated as 2.11 × 10-7 m for Wt sPLA2-IIA, 4.47 × 10-8 m for H47Q, and 1.08 × 10-6 m for R74E/R100E. d, effect of sPLA2-IIA mutations on PLA2 activity. PLA2 activity was measured as described under “Experimental Procedures.” A similar result was obtained from another independent experiment.