Abstract
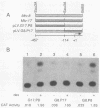
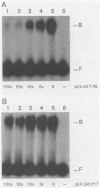
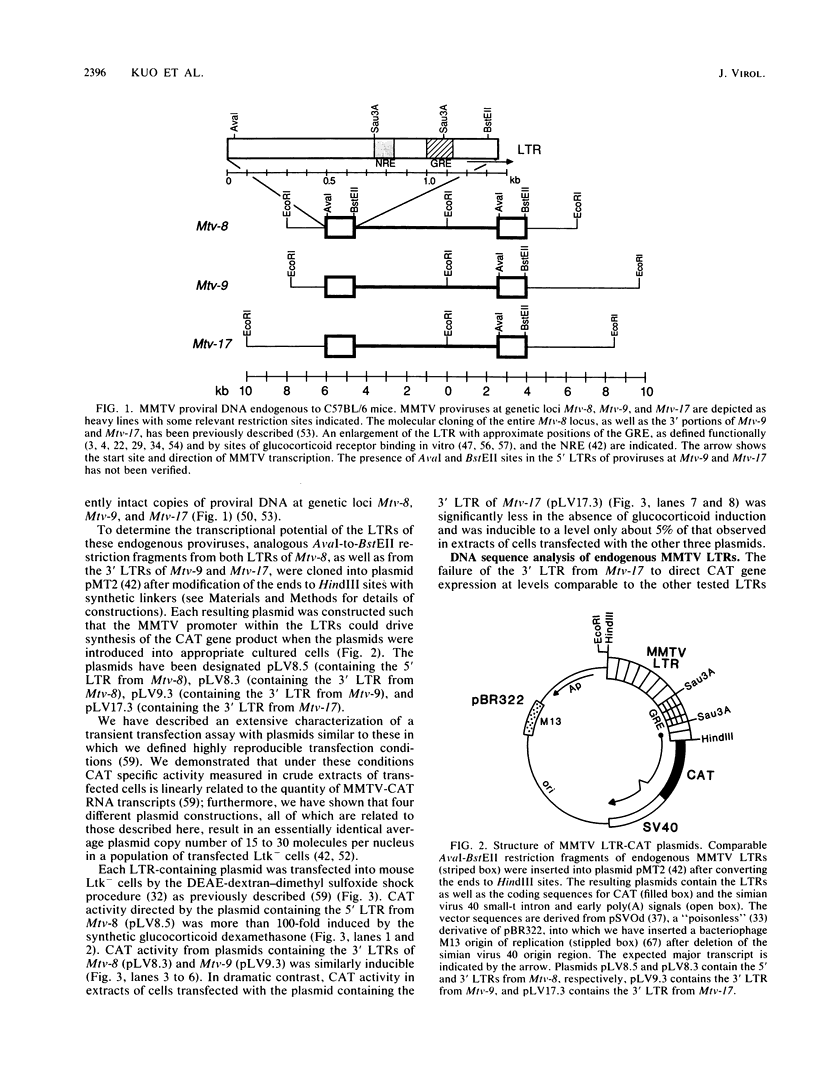
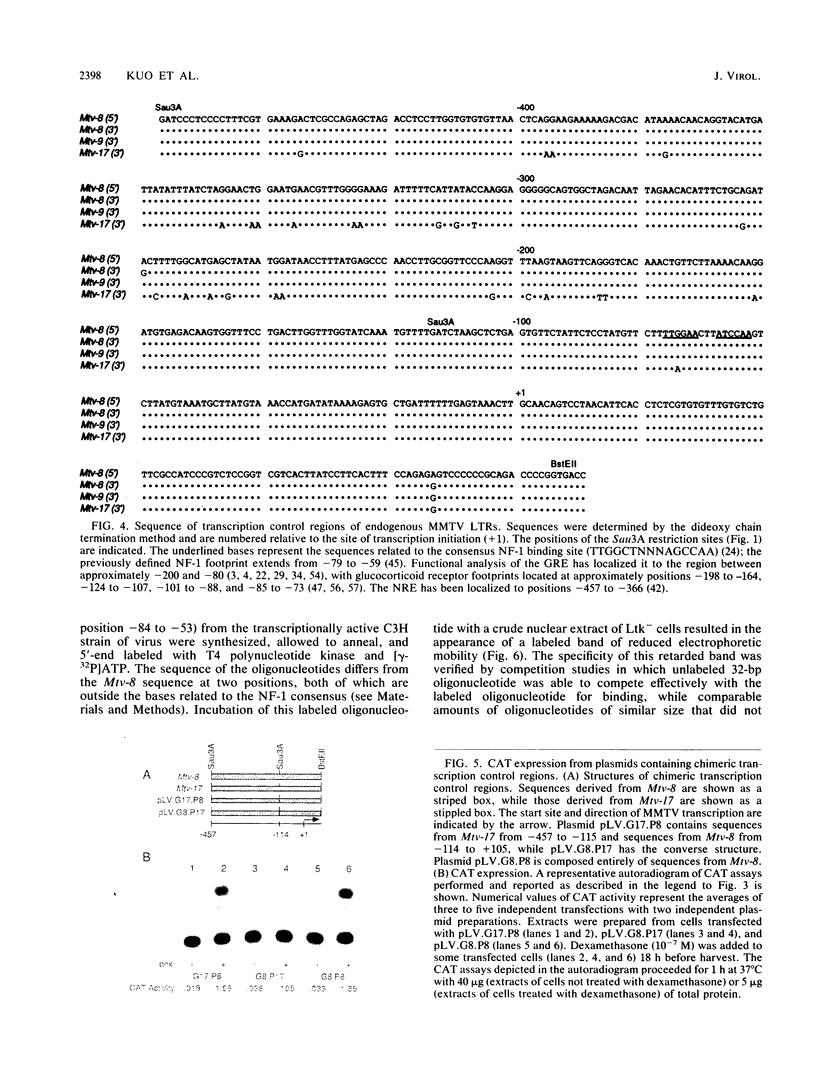
Mouse mammary tumor virus proviral DNA is endogenous to most inbred strains of mice but in many strains is not transcriptionally active. This inactivity may be due to defects in the proviruses themselves or to position effects mediated by DNA sequences flanking the proviral units. The transcriptional competence of long terminal repeats (LTRs) derived from endogenous proviral DNA at genetic loci Mtv-8, Mtv-9, and Mtv-17 of the C57BL/6 mouse strain was examined with a transient transfection assay in which gene expression was monitored by expression of chloramphenicol acetyltransferase. LTRs from Mtv-8 and Mtv-9 were able to direct glucocorticoid-induced chloramphenicol acetyltransferase expression in this assay, while the LTR from Mtv-17 was only about 5% as effective. Analysis of chimeric LTRs indicated that the glucocorticoid-inducible transcriptional enhancer element within the Mtv-17 LTR is active when linked to a functional promoter from Mtv-8, whereas the promoter from Mtv-17 is defective in directing hormone-induced gene expression, even when linked to the Mtv-8 glucocorticoid-responsive enhancer. The DNA sequence of transcriptional control regions of the LTRs of all three endogenous proviral units was determined; this analysis revealed that the source of the defect in Mtv-17 is a single G-to-A transition at position-75 with respect to the site of transcription initiation that resides within the previously defined binding site for the transcription factor nuclear factor 1. Competition experiments with a gel electrophoresis mobility shift assay indicated that the affinity of nuclear factor 1 for DNA derived from Mtv-17 is significantly less than for comparable sequences derived from Mtv-8.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buetti E., Kühnel B. Distinct sequence elements involved in the glucocorticoid regulation of the mouse mammary tumor virus promoter identified by linker scanning mutagenesis. J Mol Biol. 1986 Aug 5;190(3):379–389. doi: 10.1016/0022-2836(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Diggelmann H., Vessaz A. L., Buetti E. Cloned endogenous mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Virology. 1982 Oct 30;122(2):332–341. doi: 10.1016/0042-6822(82)90233-1. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan M. V., Spiess E., Majors J. Purified glucocorticoid receptor-hormone complex from rat liver cytosol binds specifically to cloned mouse mammary tumor virus long terminal repeats in vitro. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5157–5161. doi: 10.1073/pnas.79.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Adhya S., Nagata K., Guggenheimer R. A., Hurwitz J. Site-specific DNA binding of nuclear factor I: analyses of cellular binding sites. Mol Cell Biol. 1985 May;5(5):964–971. doi: 10.1128/mcb.5.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzburg W. H., Groner B. The chromosomal integration site determines the tissue-specific methylation of mouse mammary tumour virus proviral genes. EMBO J. 1984 May;3(5):1129–1135. doi: 10.1002/j.1460-2075.1984.tb01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Fanning T. G., Cardiff R. D. Mouse mammary tumor virus: specific methylation patterns of proviral DNA in normal mouse tissues. J Virol. 1984 Jan;49(1):66–71. doi: 10.1128/jvi.49.1.66-71.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Hall C. V., Ringold G. M., Dobson D. E., Luh J., Jacob P. E. Functional analysis of the steroid hormone control region of mouse mammary tumor virus. Nucleic Acids Res. 1984 May 25;12(10):4191–4206. doi: 10.1093/nar/12.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. A., Dumaswala U. J., Tancin S. L., Vaidya A. B. Organization and expression of endogenous murine mammary tumor virus genes in mice congenic at the H-2 complex. Virology. 1980 May;103(1):167–177. doi: 10.1016/0042-6822(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Sarkar N. H. Quantitative of murine mammary tumor virus-related RNA in mammary tissues of low- and high-mammary-tumor-incidence mouse strains. J Virol. 1981 Oct;40(1):87–95. doi: 10.1128/jvi.40.1.87-95.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K. L., Toohey M. G., Peterson D. O. Transcriptional repression of a hormone-responsive promoter. Nucleic Acids Res. 1987 Sep 11;15(17):6973–6989. doi: 10.1093/nar/15.17.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowock J., Borgmeyer U., Püschel A. W., Rupp R. A., Sippel A. E. The TGGCA protein binds to the MMTV-LTR, the adenovirus origin of replication, and the BK virus enhancer. Nucleic Acids Res. 1985 Mar 25;13(6):2045–2061. doi: 10.1093/nar/13.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., de Moes J., Hilkens J., van Nie R. Localization of a gene for expression of mouse mammary tumor virus antigens in the GR/Mtv-2- mouse strain. J Exp Med. 1980 Sep 1;152(3):712–719. doi: 10.1084/jem.152.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Payvar F., Firestone G. L., Ross S. R., Chandler V. L., Wrange O., Carlstedt-Duke J., Gustafsson J. A., Yamamoto K. R. Multiple specific binding sites for purified glucocorticoid receptors on mammary tumor virus DNA. J Cell Biochem. 1982;19(3):241–247. doi: 10.1002/jcb.240190305. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Placzek M., Brookes S., Kozak C., Smith R., Dickson C. Characterization, chromosome assignment, and segregation analysis of endogenous proviral units of mouse mammary tumor virus. J Virol. 1986 Sep;59(3):535–544. doi: 10.1128/jvi.59.3.535-544.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. O. Alterations in chromatin structure associated with glucocorticoid-induced expression of endogenous mouse mammary tumor virus genes. Mol Cell Biol. 1985 May;5(5):1104–1110. doi: 10.1128/mcb.5.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. O., Beifuss K. K., Morley K. L. Context-dependent gene expression: cis-acting negative effects of specific procaryotic plasmid sequences on eucaryotic genes. Mol Cell Biol. 1987 Apr;7(4):1563–1567. doi: 10.1128/mcb.7.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. O., Kriz K. G., Marich J. E., Toohey M. G. Sequence organization and molecular cloning of mouse mammary tumor virus DNA endogenous to C57BL/6 mice. J Virol. 1985 May;54(2):525–531. doi: 10.1128/jvi.54.2.525-531.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Kennedy N., Skroch P., Hynes N. E., Groner B. Hormonal response region in the mouse mammary tumor virus long terminal repeat can be dissociated from the proviral promoter and has enhancer properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1020–1024. doi: 10.1073/pnas.82.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Toohey M. G., Morley K. L., Peterson D. O. Multiple hormone-inducible enhancers as mediators of differential transcription. Mol Cell Biol. 1986 Dec;6(12):4526–4538. doi: 10.1128/mcb.6.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Firestone G. L., Yamamoto K. R. Glucocorticoids and chromosomal position modulate murine mammary tumor virus transcription by affecting efficiency of promoter utilization. Mol Cell Biol. 1983 Apr;3(4):551–561. doi: 10.1128/mcb.3.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Taraschi N. E., Tancin S. L., Long C. A. Regulation of endogenous murine mammary tumor virus expression in C57BL mouse lactating mammary glands: transcription of functional mRNA with a block at the translational level. J Virol. 1983 Jun;46(3):818–828. doi: 10.1128/jvi.46.3.818-828.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nie R., Verstraeten A. A., De Moes J. Genetic transmission of mammary tumour virus by GR mice. Int J Cancer. 1977 Mar 15;19(3):383–390. doi: 10.1002/ijc.2910190316. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Verstraeten A. A., van Nie R. Genetic transmission of mammary tumour virus in the DBAf mouse strain. Int J Cancer. 1978 Apr 15;21(4):473–475. doi: 10.1002/ijc.2910210412. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]