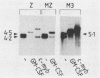
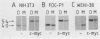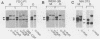Abstract
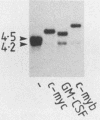
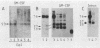
In order to identify factors that influence expression by retroviral vectors in hemopoietic cells, we have compared viral RNA levels in cells infected with several different recombinant viruses. All of the vectors tested carry the neomycin resistance gene and provide for the insertion of a second gene which, in these studies, comprised sequences from the myc or myb oncogenes or the gene encoding granulocyte-macrophage colony-stimulating factor. The vectors utilize two different strategies for the coexpression of the two genes: alternate splicing and the use of a separate internal promoter. We found that expression in hemopoietic cells could be increased by substituting sequences from the myeloproliferative sarcoma virus long terminal repeat for those of the Moloney murine leukemia virus long terminal repeat. However, none of the vectors examined was able to express a second gene at levels equivalent to those achieved by the parental vectors carrying only the neomycin resistance gene. The reasons for this varied with the different vectors and included inefficient splicing and/or a reduction in the level of unspliced transcripts upon insertion of a second gene. Although the basis of the latter phenomenon is not clear, it is probably related to the position--near the 5' long terminal repeat--at which the second gene was inserted, since insertion of the same genes near the 3' end of another vector had no effect on viral RNA levels. In an attempt to circumvent some of these problems, we constructed a vector that employs an internal beta-actin promoter. Although this vector could express granulocyte-macrophage colony-stimulating factor sequences in a responsive hemopoietic cell line, the level of granulocyte-macrophage colony-stimulating factor produced was disappointingly low. The results from these studies suggest approaches to the design of improved vectors for effective expression of genes in hemopoietic cells.
Full text
PDF
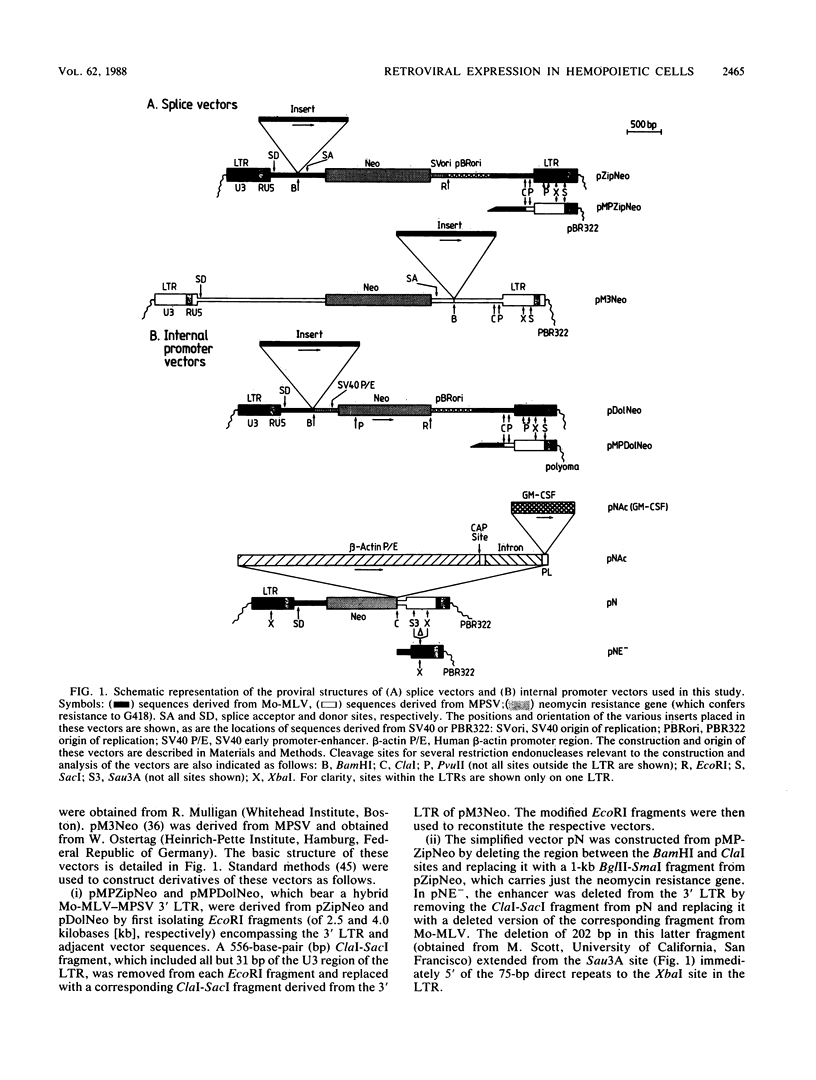








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Baumbach W. R., Keath E. J., Cole M. D. A mouse c-myc retrovirus transforms established fibroblast lines in vitro and induces monocyte-macrophage tumors in vivo. J Virol. 1986 Aug;59(2):276–283. doi: 10.1128/jvi.59.2.276-283.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont J. W., Henkel-Tigges J., Chang S. M., Wager-Smith K., Kellems R. E., Dick J. E., Magli M. C., Phillips R. A., Bernstein A., Caskey C. T. Expression of human adenosine deaminase in murine haematopoietic progenitor cells following retroviral transfer. Nature. 1986 Jul 24;322(6077):385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Bowtell D. D. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987 May 1;162(2):463–465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Rous sarcoma virus encodes a transcriptional activator. Cell. 1985 Mar;40(3):537–546. doi: 10.1016/0092-8674(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Bernard O., Bowtell D., Schrader S., Schrader J. W. Murine c-myc retroviruses alter the growth requirements of myeloid cell lines. Oncogene Res. 1987 Jun;1(1):61–76. [PubMed] [Google Scholar]
- Cory S., Gerondakis S., Adams J. M. Interchromosomal recombination of the cellular oncogene c-myc with the immunoglobulin heavy chain locus in murine plasmacytomas is a reciprocal exchange. EMBO J. 1983;2(5):697–703. doi: 10.1002/j.1460-2075.1983.tb01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. A promoterless retroviral vector indicates that there are sequences in U3 required for 3' RNA processing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1197–1201. doi: 10.1073/pnas.84.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Gough N. M., Dunn A. R., de Blaquiere J. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 1985 Aug;4(8):2003–2008. doi: 10.1002/j.1460-2075.1985.tb03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. H., Gilboa E. Expression of genes introduced into cells by retroviral infection is more efficient than that of genes introduced into cells by DNA transfection. J Virol. 1984 May;50(2):417–424. doi: 10.1128/jvi.50.2.417-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. S., Park J., Gilboa E. Role of intron-contained sequences in formation of moloney murine leukemia virus env mRNA. Mol Cell Biol. 1984 Nov;4(11):2289–2297. doi: 10.1128/mcb.4.11.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Nature of cells forming erythroid colonies in agar after stimulation by spleen conditioned medium. J Cell Physiol. 1978 Mar;94(3):243–252. doi: 10.1002/jcp.1040940302. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Bernstein A. Retrovirus transduction: segregation of the viral transforming function and the herpes simplex virus tk gene in infectious Friend spleen focus-forming virus thymidine kinase vectors. Mol Cell Biol. 1983 Dec;3(12):2191–2202. doi: 10.1128/mcb.3.12.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A., Keller G., Phillips R. A., Bernstein A. Retrovirus transfer of a bacterial gene into mouse haematopoietic progenitor cells. Nature. 1983 Oct 6;305(5934):556–558. doi: 10.1038/305556a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Kollek R., Stocking C., Smadja-Joffe F., Ostertag W. Molecular cloning and characterization of a leukemia-inducing myeloproliferative sarcoma virus and two of its temperature-sensitive mutants. J Virol. 1984 Jun;50(3):717–724. doi: 10.1128/jvi.50.3.717-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Proviral sequences that restrict retroviral expression in mouse embryonal carcinoma cells. Mol Cell Biol. 1987 Oct;7(10):3775–3784. doi: 10.1128/mcb.7.10.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Magli M. C., Dick J. E., Huszar D., Bernstein A., Phillips R. A. Modulation of gene expression in multiple hematopoietic cell lineages following retroviral vector gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(3):789–793. doi: 10.1073/pnas.84.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R. Production by spleen and lymph node cells of conditioned medium with erythroid and other hemopoietic colony-stimulating activity. J Cell Physiol. 1978 Jul;96(1):31–42. doi: 10.1002/jcp.1040960105. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Autoinduction of differentiation in WEHI-3B leukemia cells. Int J Cancer. 1982 Dec 15;30(6):773–780. doi: 10.1002/ijc.2910300616. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. Insertion of several different DNAs in reticuloendotheliosis virus strain T suppresses transformation by reducing the amount of subgenomic mRNA. J Virol. 1986 Apr;58(1):75–80. doi: 10.1128/jvi.58.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. M., Bader J. P., Bassin R. H. Inhibitors of glycosylation reverse retroviral interference. Virology. 1982 May;119(1):185–192. doi: 10.1016/0042-6822(82)90075-7. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stacey A., Arbuthnott C., Kollek R., Coggins L., Ostertag W. Comparison of myeloproliferative sarcoma virus with Moloney murine sarcoma virus variants by nucleotide sequencing and heteroduplex analysis. J Virol. 1984 Jun;50(3):725–732. doi: 10.1128/jvi.50.3.725-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Schuetze S., Vanek M., Wagner E. F. Expression of retroviral vectors in transgenic mice obtained by embryo infection. EMBO J. 1987 Feb;6(2):383–388. doi: 10.1002/j.1460-2075.1987.tb04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Long terminal repeat sequences impart hematopoietic transformation properties to the myeloproliferative sarcoma virus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5746–5750. doi: 10.1073/pnas.82.17.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Point mutations in the U3 region of the long terminal repeat of Moloney murine leukemia virus determine disease specificity of the myeloproliferative sarcoma virus. Virology. 1986 Aug;153(1):145–149. doi: 10.1016/0042-6822(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Weiher H., Barklis E., Ostertag W., Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987 Sep;61(9):2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y. P., Moore M. A. Long-term in vitro culture of murine mast cells. III. Discrimination of mast cells growth factor and granulocyte-CSF. J Immunol. 1982 Sep;129(3):1256–1261. [PubMed] [Google Scholar]