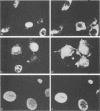
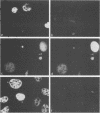
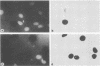
Abstract
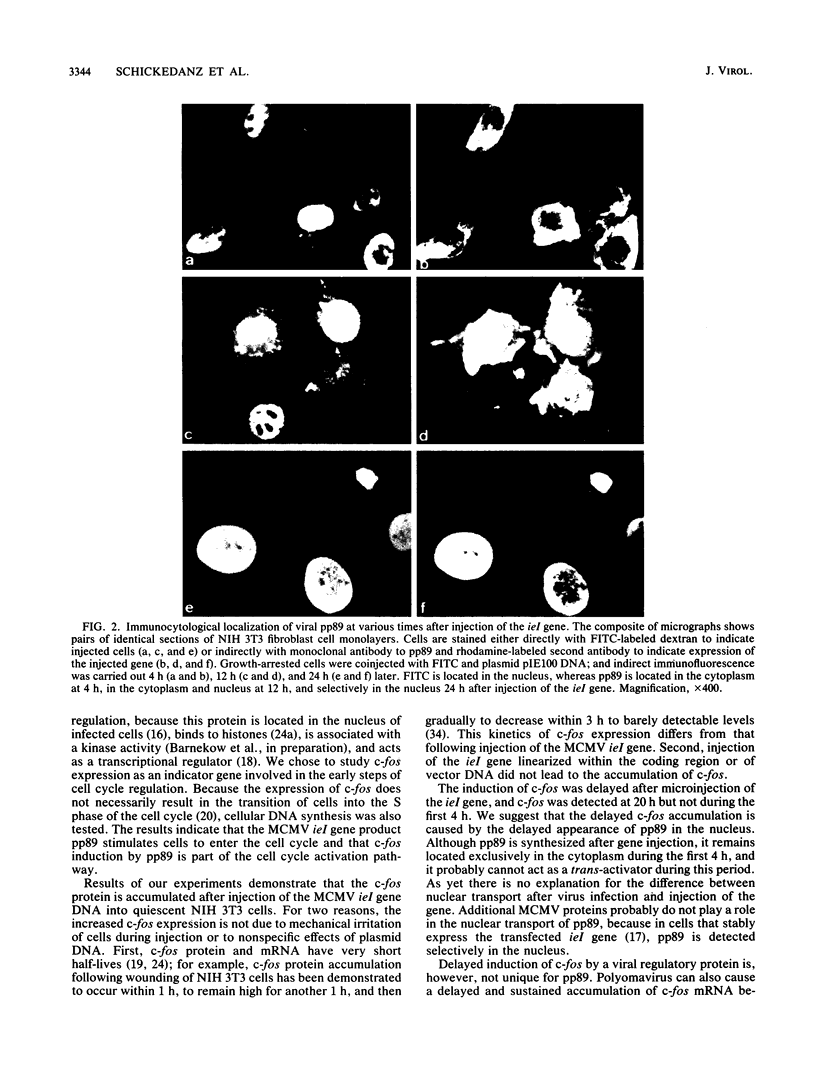
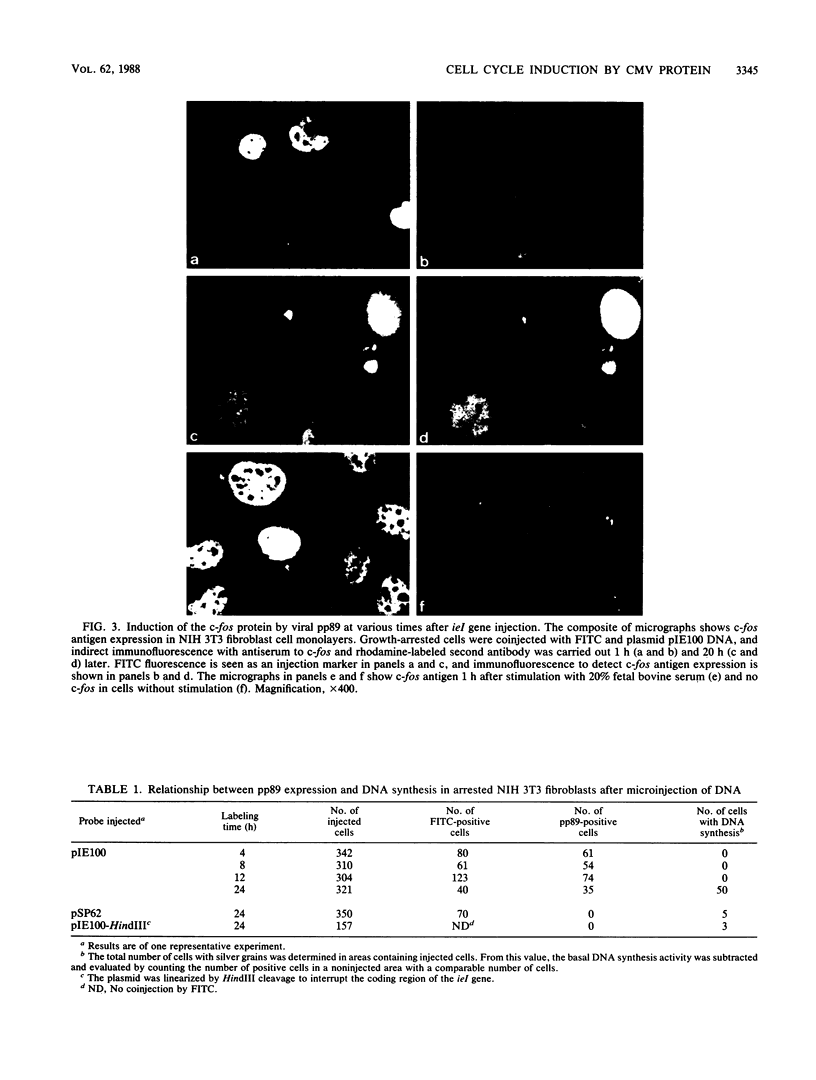
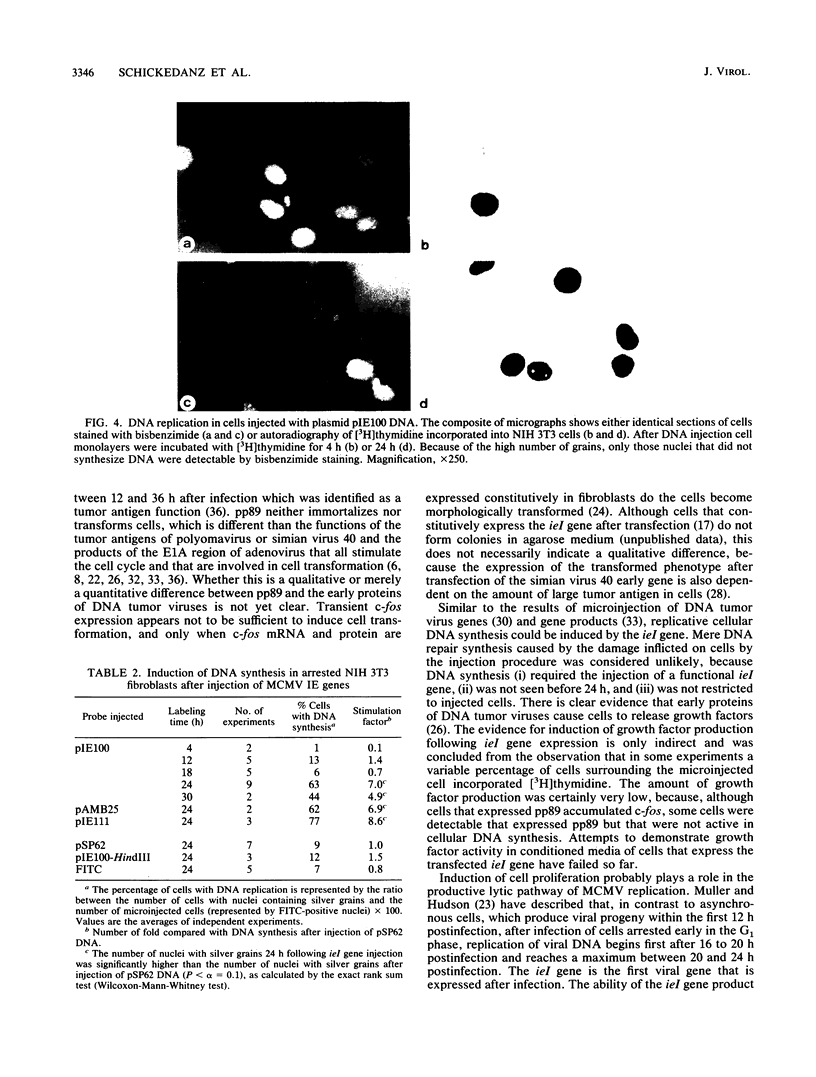
The immediate-early (IE) genes of murine cytomegalovirus (MCMV) are expressed in the absence of prior viral protein synthesis and regulate the transcription of MCMV early genes. The effect of MCMV IE genes on growth induction was studied. Different plasmids containing MCMV IE genes were microinjected into arrested NIH 3T3 mouse fibroblasts. Plasmids containing the ieI gene coding for the 89,000-Mr major IE protein pp89 were found to stimulate the expression of the c-fos protooncogene. Synthesis of pp89 and its transport to the nucleus appeared to be required for c-fos expression. DNA synthesis occurred in cells that were injected with MCMV IE genes and in neighboring cells that were not injected. The results suggest that the phosphoprotein pp89 stimulates cells to enter the cell cycle.
Full text
PDF

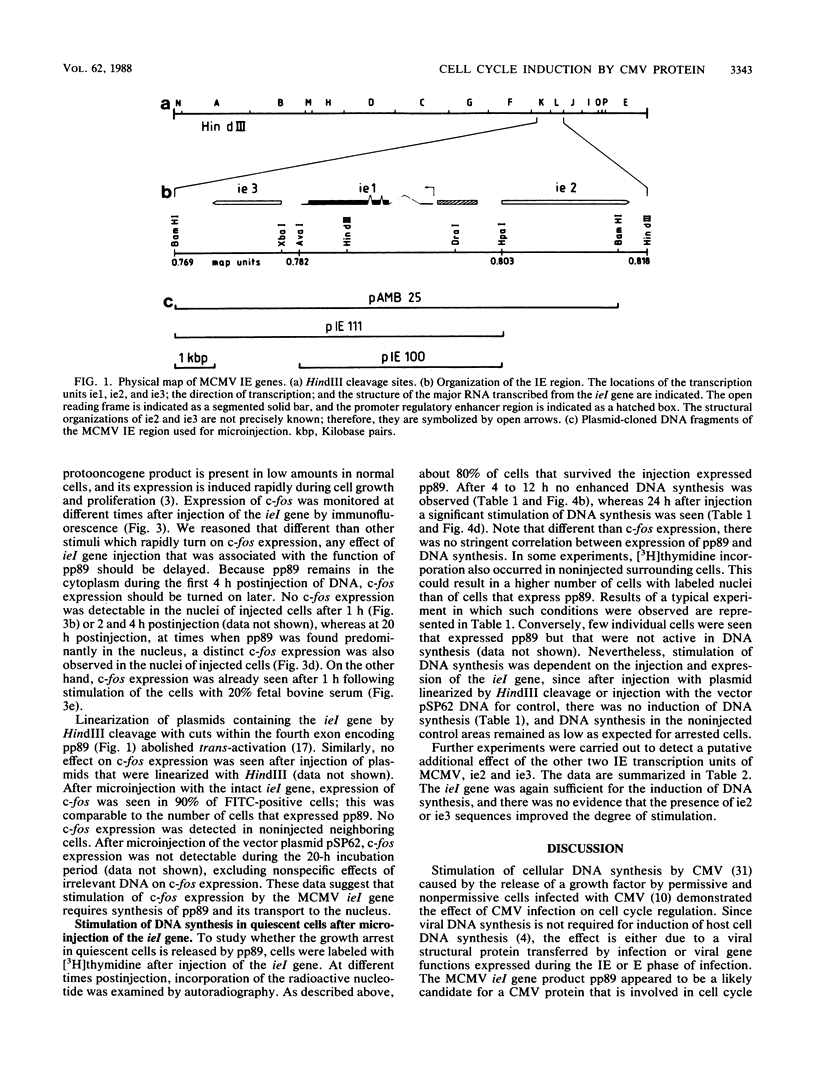

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W. Improved system for capillary microinjection into living cells. Exp Cell Res. 1982 Jul;140(1):31–37. doi: 10.1016/0014-4827(82)90152-5. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. The role of defective cytomegalovirus particles in the induction of host cell DNA synthesis. Virology. 1977 Oct 1;82(1):93–99. doi: 10.1016/0042-6822(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Keil G. M., Weber F., Jasin M., Schaffner W., Koszinowski U. H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A., Keil G. M., Knust E., Koszinowski U. H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983 Sep;47(3):421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Fleckenstein B., Hennighausen L. Binding of transcription factors and creation of a large nucleoprotein complex on the human cytomegalovirus enhancer. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3658–3662. doi: 10.1073/pnas.84.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczöl E., Plotkin S. A. Cells infected with human cytomegalovirus release a factor(s) that stimulates cell DNA synthesis. J Gen Virol. 1984 Oct;65(Pt 10):1833–1837. doi: 10.1099/0022-1317-65-10-1833. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Immediate-early genes of murine cytomegalovirus: location, transcripts, and translation products. J Virol. 1987 Feb;61(2):526–533. doi: 10.1128/jvi.61.2.526-533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Fibi M. R., Koszinowski U. H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985 May;54(2):422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Keil G. M., Schwarz H., Schickedanz J., Reddehase M. J. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J Exp Med. 1987 Jul 1;166(1):289–294. doi: 10.1084/jem.166.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Keil G. M., Volkmer H., Fibi M. R., Ebeling-Keil A., Münch K. The 89,000-Mr murine cytomegalovirus immediate-early protein activates gene transcription. J Virol. 1986 Apr;58(1):59–66. doi: 10.1128/jvi.58.1.59-66.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. G., Gray H. E., Tötterman T., Pettersson U., Nilsson K. Drastically increased expression of MYC and FOS protooncogenes during in vitro differentiation of chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):223–227. doi: 10.1073/pnas.84.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Cell cycle dependency of murine cytomegalovirus replication in synchronized 3T3 cells. J Virol. 1977 May;22(2):267–272. doi: 10.1128/jvi.22.2.267-272.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Münch K., Keil G. M., Messerle M., Koszinowski U. H. Interaction of the 89K murine cytomegalovirus immediate-early protein with core histones. Virology. 1988 Apr;163(2):405–412. doi: 10.1016/0042-6822(88)90281-4. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Sullivan N., Grodzicker T. Growth factor(s) produced during infection with an adenovirus variant stimulates proliferation of nonestablished epithelial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3283–3287. doi: 10.1073/pnas.84.10.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Yamaguchi N. Induction of c-Ha-ras transcription in rat cells by simian virus 40 large T antigen. Mol Cell Biol. 1987 Jan;7(1):556–559. doi: 10.1128/mcb.7.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Ogino T., Baba K., Onaka M. Synthesis of deoxyribonucleic acid in human and hamster kidney cells infected with human adenovirus types 5 and 12. Virology. 1969 Apr;37(4):513–520. doi: 10.1016/0042-6822(69)90269-4. [DOI] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier B., Müller D., Bravo R., Müller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986 May;5(5):913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J., Stiles C. D., Garcea R. L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]