Abstract
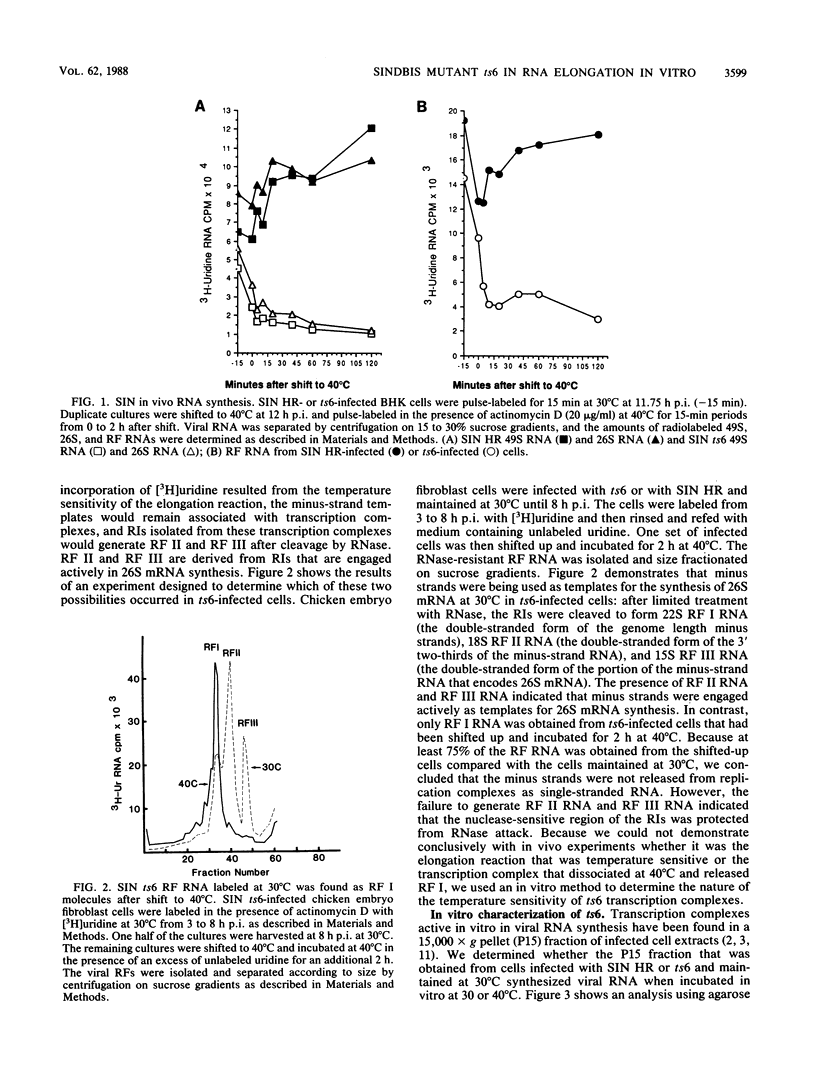
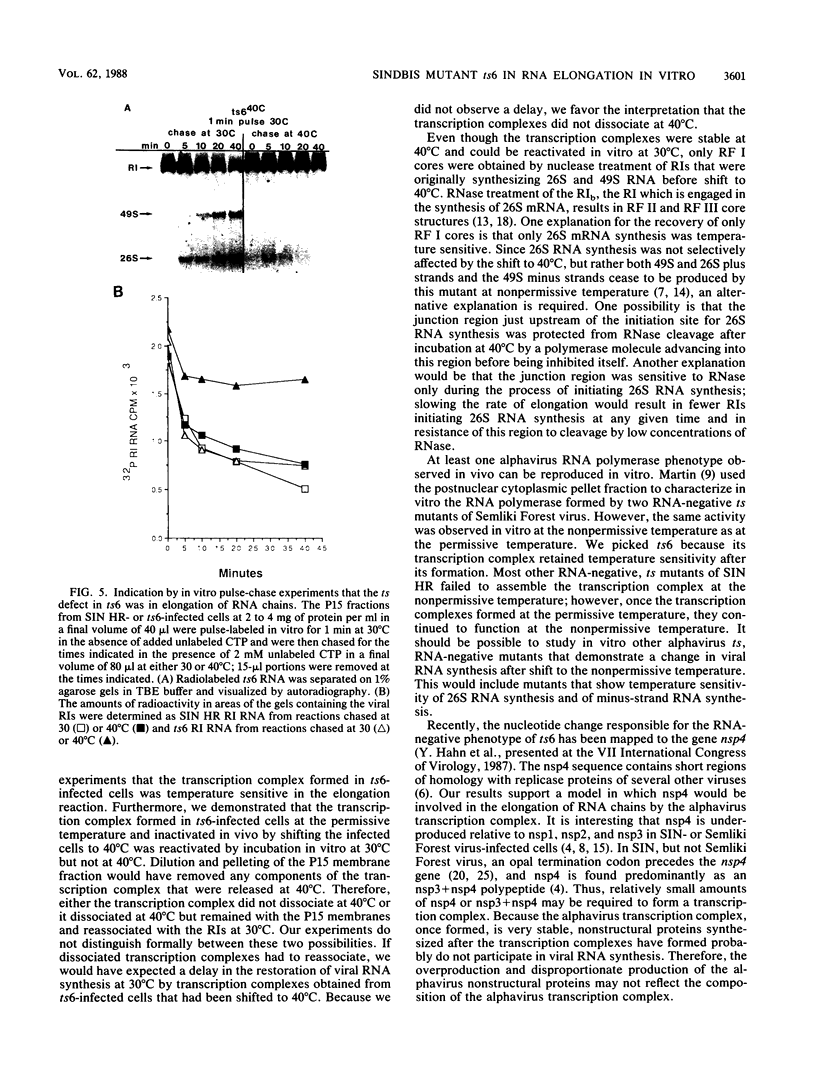
Characterization of conditionally lethal mutants of alphaviruses, Sindbis virus and Semliki Forest virus, has indicated that in almost all the RNA-negative mutants the temperature-sensitive (ts) defect prevents the formation of active transcription complexes at nonpermissive temperature (40 degrees C), but such complexes retain activity at 40 degrees C if formed first at permissive temperature (30 degrees C). Our recent results have extended the characterization of one exception to this finding: Sindbis ts6 transcription complexes, once formed at 30 degrees C, do not function at 40 degrees C. We used an in vitro assay for viral RNA synthesis to determine whether the ts defect was the result of dissociation of the complex or of a failure to elongate RNA chains in a stable complex. Our results indicated that the phenotype of ts6 observed in vivo was retained in vitro. In vivo incorporation into single-stranded 49S and 26S RNA was inhibited simultaneously with its incorporation into replicative intermediates upon shifting ts6-infected cells to 40 degrees C, which was compatible with a defect in elongation. Complexes formed at 30 degrees C and inactivated in vivo by shifting to 40 degrees C were reactivated by incubation in vitro at 30 degrees C but not at 40 degrees C. Thus, the transcription complexes were stable. Nascent RNA chains initiated in vivo and pulse-labeled in vitro were chased into single-stranded 49S and 26S RNA only when incubation was at 30 degrees C, indicating that the ts6 transcription complex was temperature sensitive in elongation. It should be possible to study in vitro other alphavirus RNA-negative mutants that demonstrate a change in viral RNA synthesis after shift to 40 degrees C. These would include ts mutants in the synthesis of subgenomic 26S mRNA and of minus-strand RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Käriäinen L., Keränen S., Ranki M., Sawicki D. L. Semliki Forest virus replication complex capable of synthesizing 42S and 26S nascent RNA chains. J Gen Virol. 1980 Jul;49(1):61–69. doi: 10.1099/0022-1317-49-1-61. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Ruohonen L. Nonstructural proteins of Semliki Forest virus: synthesis, processing, and stability in infected cells. J Virol. 1983 Sep;47(3):505–515. doi: 10.1128/jvi.47.3.505-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. M. Studies on the RNA polymrase of some temperature-sensitive mutants of Semliki Forest virus. Virology. 1969 Sep;39(1):107–117. doi: 10.1016/0042-6822(69)90352-3. [DOI] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979 Oct 30;98(2):298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Kaariainen L., Lambek C., Gomatos P. J. Mechanism for control of synthesis of Semliki Forest virus 26S and 42s RNA. J Virol. 1978 Jan;25(1):19–27. doi: 10.1128/jvi.25.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of overproduction of nonstructural proteins on alphavirus plus-strand and minus-strand RNA synthesis. Virology. 1986 Jul 30;152(2):507–512. doi: 10.1016/0042-6822(86)90157-1. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Sequence coding for the alphavirus nonstructural proteins is interrupted by an opal termination codon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5271–5275. doi: 10.1073/pnas.80.17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Kuhn R. J., Yang C. F., Takegami T., Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986 Oct;60(1):43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K. Complete nucleotide sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 1986 Jul 25;14(14):5667–5682. doi: 10.1093/nar/14.14.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]