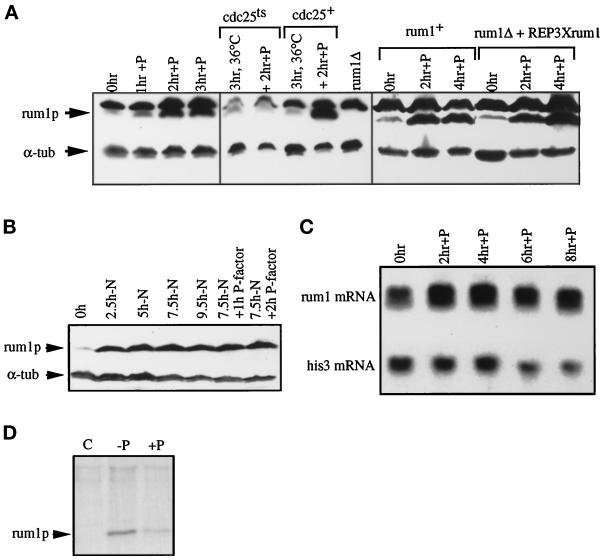
Figure 2.
rum1p is induced in G1 in pheromone. (A) Western blot of extracts from P-factor–treated cells probed with anti-rum1p and control anti-α-tubulin antibodies. A lane with extracts from isogenic rum1Δ cells demonstrates that the slower migrating band cross-reacting with anti-rum1p is nonspecific. A cyr1Δsxa2Δ strain exposed to P-factor for 3 h at 25°C (left panel), cdc25–22cyr1Δsxa2Δ and cyr1Δsxa2Δ strains incubated at 36°C for 3 h and exposed to P-factor for 2 h at 36°C (middle panel), and a rum1Δcyr1Δsxa2Δ::REP6Xrum1+ integrant and a rum1+ control exposed to P-factor at 25°C for 4 h (right panel). (B) Cyr1Δsxa2Δ cells were nitrogen starved, and after 7.5 h, P-factor was added to half the culture. Extracts were Western blotted and probed with anti-rum1p and anti-α-tubulin antibodies. This anti-rum1p antibody shows no nonspecific cross-reacting band. (C) Northern blot of RNA samples from a cyr1Δsxa2Δ strain exposed to P-factor for 8 h at 29°C, probed for rum1+ and his3+ mRNA. (D) Protein extracts from 35S-methionine pulse-labeled cyr1Δsxa2Δ cells before (C, −P) or after 3 h exposure to P-factor (+P), immunoprecipitated with preimmune serum (C), or anti-rum1p antibody (−P and +P). Because there was considerably more 35S-methionine–labeled protein in the −P extract than in the +P extract, the rum1p-specific band was quantified and normalized with respect to an unspecific band. With this quantification, 120 relative units of 35S were incorporated in the absence of pheromone, compared with 97 relative units in the presence of pheromone.