Figure 7.
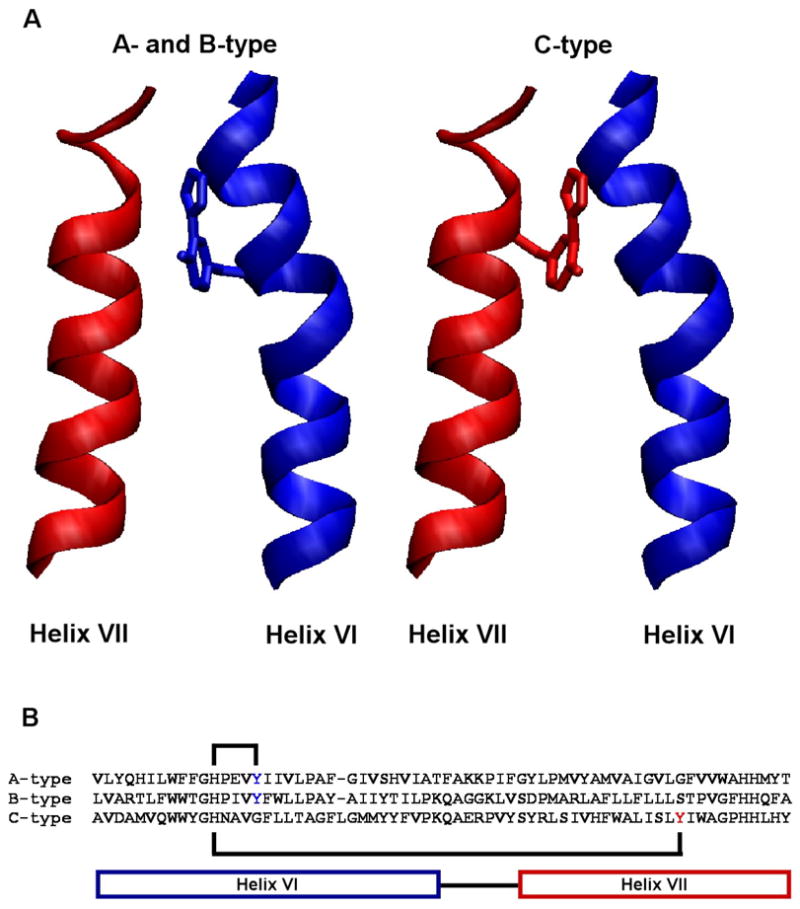
A novel crosslinked cofactor is present in all three heme-copper oxygen reductase families. (A) The active-site tyrosine forming the cofactor originates from helix VI in the A- and B-type oxygen reductases, while in the C-type oxygen reductases it originates from helix VII. This is the first example of the evolutionary migration of a post-translationally modified active-site residue. (B) The crosslink is formed between a histidine and tyrosine within helix VI in the A- and B-type oxygen reductases. In the C-type oxygen reductases the crosslink is formed between a histidine in helix VI and a tyrosine in helix VII, covalently coupling the helices together. This figure was generated using VMD software (45).
