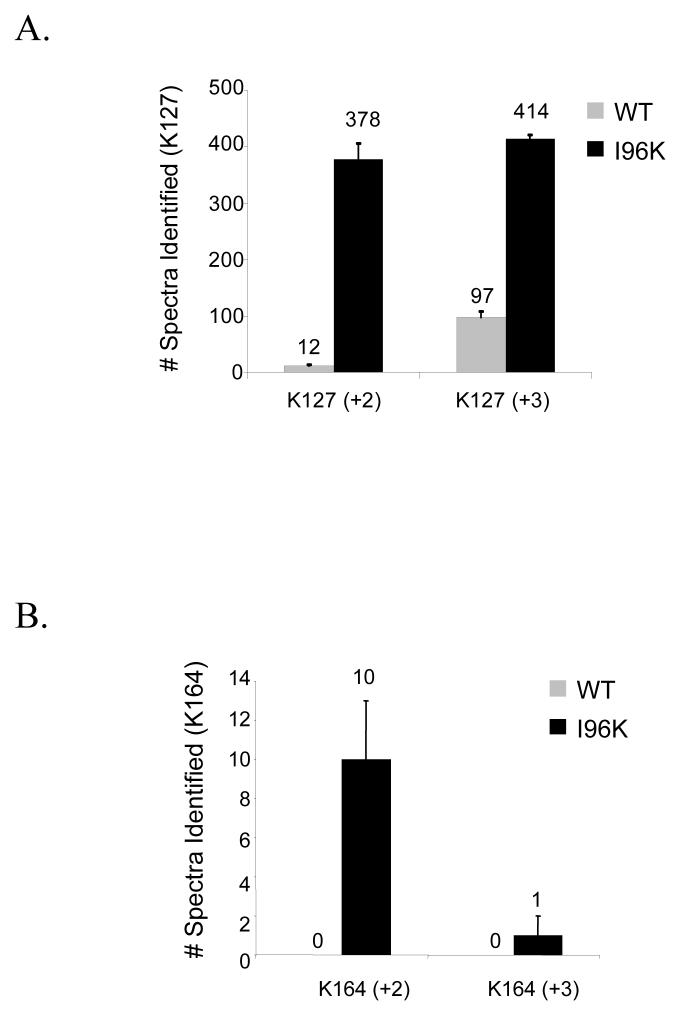
Figure 3. Comparison of mass spectrometry-based identification of Smt3p-attachment sites in Pol30p when conjugated to either wild-type Smt3p or Smt3p-I96K.
(A-C) The identification of peptides (+2 and +3 charge states) spanning the (A) K127 and (B) K164 sumoylation sites of GST-Pol30p is more efficient for peptides modified by Smt3p-I96K (+114 Da) than those modified by wild-type Smt3p (+484 Da) while (C) unmodified peptides are identified equally well. GST-Pol30p sumoylated by either Smt3p-WT or Smt3p-I96K was digested by trypsin and analyzed using the directed MS/MS strategy as described in the Experimental Procedures. Spectra were identified using SEQUEST considering appropriate masses for the modified lysine. The number of peptide identifications shown is the average of three independent experiments.
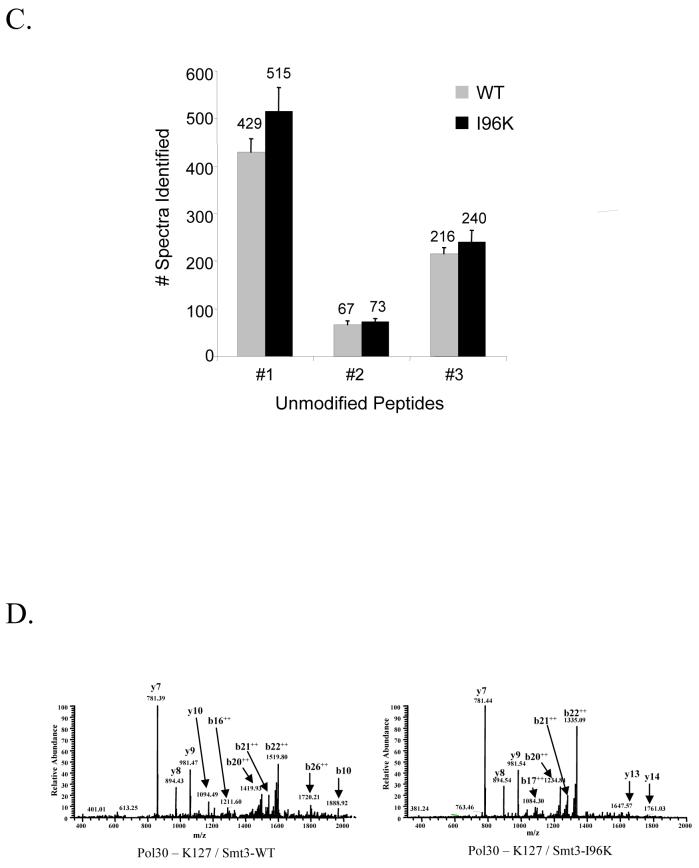
(D) Representative spectra of the sumoylated peptides spanning the K127 site of Pol30p and containing either the -EQIGG tag from Smt3p-WT (left) or the -GG from Smt3p-I96K (right).
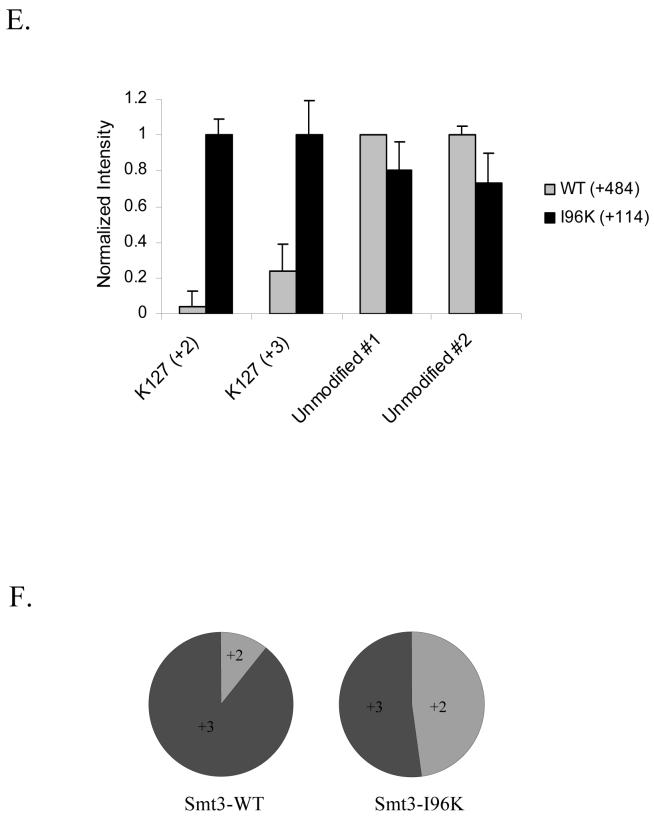
(E) Normalized intensities of Pol30p-K127 sumoylated peptides and unmodified control peptides derived from GST-Pol30p conjugated to either Smt3p-WT or Smt3p-I96K. Intensity values are the average of three independent experiments.
(F) Graph showing the ratio of +2 to +3 spectra for sumoylated peptides spanning the K127 site of GST-Pol30 conjugated to either Smt3p-WT or Smt3p-I96K.