Scheme 2.
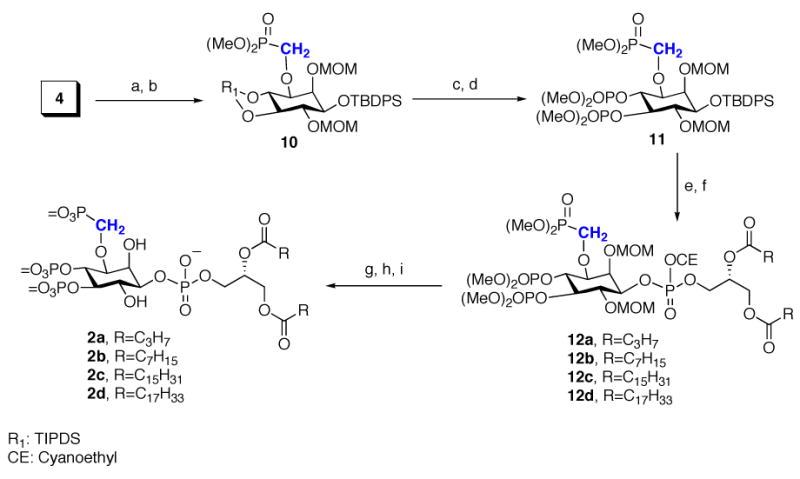
Synthesis of Methylenephosphonates 2a
a Conditions: (a) Dibal-H, CH2Cl2, −78 °C, 88%; (b) n-BuLi, HMPA, dimethyl phosphonomethyltriflate, THF, −78 °C to rt, 80%; (c) TBAF, 90%; (d) N,N-dimethylphosphoramidite, 1H -tetrazole; m-CPBA, 95%; (e) TBAF·3H2O, DMF, 75%; (f) 8a–8d, 1H -tetrazole, CH2Cl2; then t-BuOOH; (g) TEA, BSTFA, CH3CN; (h) TMSBr/CH2Cl2 (2:3); (i) MeOH.
