Figure 1.
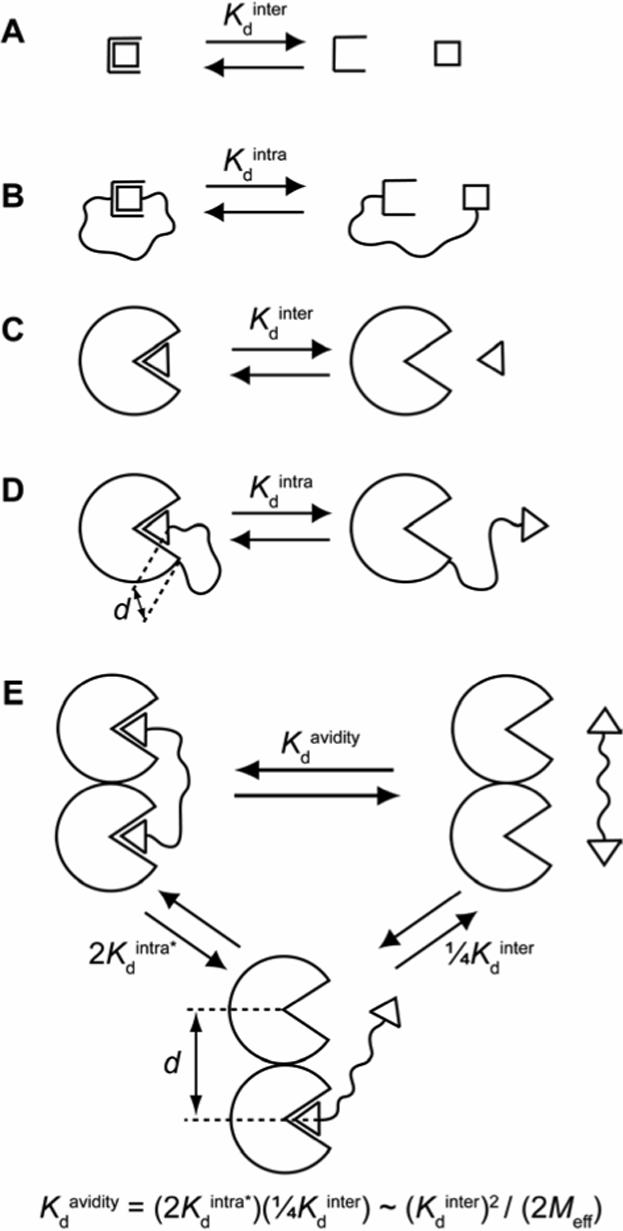
(A) A monovalent binding event between a receptor and ligand is characterized by a dissociation constant (Kdinter) with units of concentration (M). (B) When the ligand is tethered to the receptor, the dissociation constant (Kdintra) is now dimensionless and is related to Kdinter by the effective molarity, Meff (eq 1). (C) Analogous to (A) for a binding event between a protein and ligand. (D) Analogous to (B) for an intramolecular protein-ligand binding event. In this case, however, the distance (d) between the two ends of the linker in the bound state is non-zero (unlike the case in (B)). (E) Dissociation of a bivalent ligand from a bivalent receptor. This process can be conceptualized as occurring in two steps: the first step (with dissociation constant 2Kdintra*) is intramolecular, and the second step (with dissociation constant ¼Kdinter) is intermolecular. The equation given provides a means of estimating Meff from the observed dissociation constant (Kdavidity) and the intermolecular dissociation constant (Kdinter) by assuming that the dissociation constant for the first step is equivalent to the product of a statistical factor of 2 and Kdintra (defined in (D); i.e., this procedure assumes that Kdintra* = Kdintra). This assumption is often quite poor because of complications arising from the influence of binding at one site on binding at the other site (e.g., cooperativity between binding sites, enthalpy/entropy compensation).4
