Abstract
It is useful to be able to measure selection pressures acting on resistance genes in insect vectors of disease, since it is thus possible to predict future changes in frequency and to consider ways to minimize development of resistance. This note describes a method for estimating the selection coefficients, given two or more post-selection phenotype frequencies and knowing the number of generations between them.
The method is applied to published data on Anopheles labranchiae under selection with DDT. The relative fitness (1-s) of the susceptibles compared with resistants was estimated by this method to be 31-38%. This was an annual estimate, but if the number of generations per year is known, it is also possible to calculate a value per generation. A computer program for making these estimates is given. The calculations depend on the gene being effectively recessive, i.e., on the heterozygote being killed by the dose applied in the field.
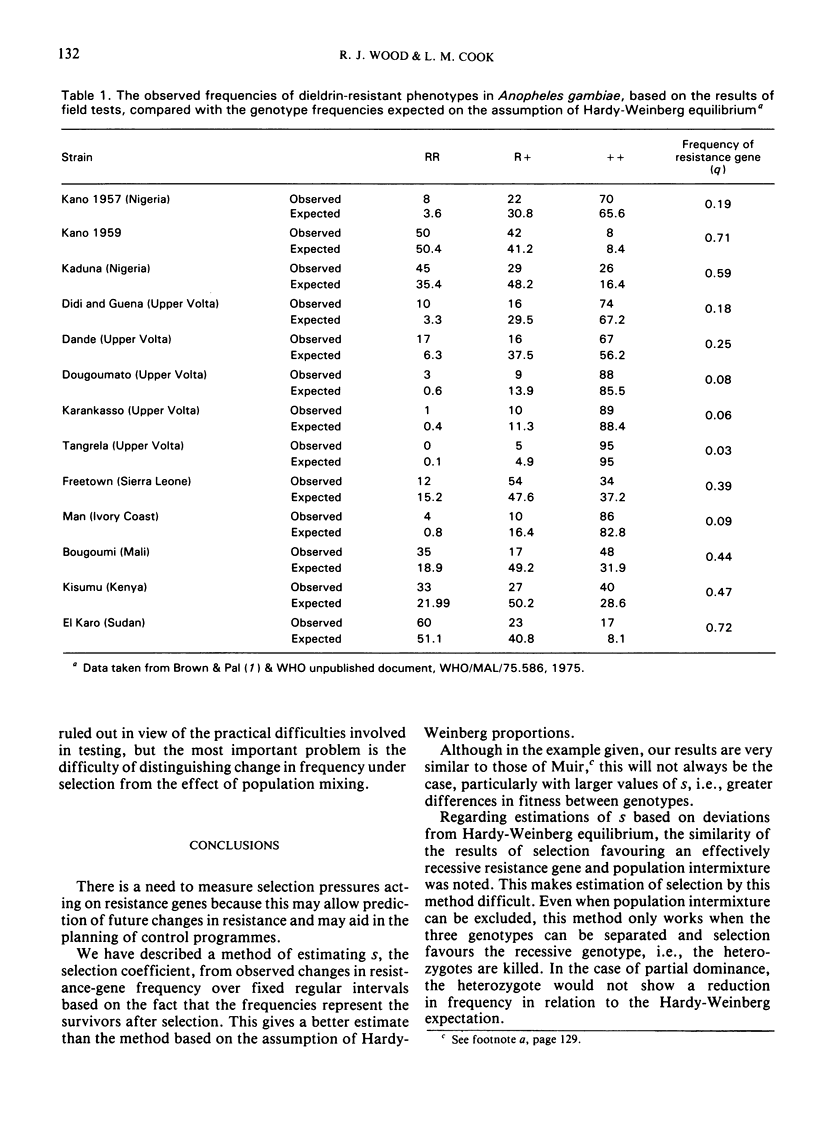
Another approach to estimation of selection is by determining the deviation in gene frequency from the Hardy-Weinberg expectations. By this method, the relative fitness (1-s) of the susceptibles in a population of A. funestris under dieldrin selection in the north of the United Republic of Cameroon has been estimated to be 40%. There are difficulties with this method, however, because population mixing may result in deviations that mimic the effect of selection. Examples are discussed for A. gambiae, where population mixing may occur and heterozygote deficiencies for the dieldrin resistance gene have been observed.
For both methods of estimation, it is essential to know the real effective dominance of the resistance gene in the wild, i.e., whether the resistance heterozygote is killed or not. This factor is important in the control of resistance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coluzzi M., Sabatini A., Petrarca V., Di Deco M. A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73(5):483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G. THE FIVE MATING-TYPES IN THE ANOPHELES GAMBIAE COMPLEX. Riv Malariol. 1964 Dec;43:167–183. [PubMed] [Google Scholar]
- DAVIDSON G. The practical implications of studies on insecticide resistance in anopheline mosquitoes. Indian J Malariol. 1958 Dec;12:413–422. [PubMed] [Google Scholar]
- Paterson H. E., Green C. A., Mahon R. J. Letter: Aedes aegypti complex in Africa. Nature. 1976 Jan 22;259(5540):252–252. doi: 10.1038/259252b0. [DOI] [PubMed] [Google Scholar]
- Scott J. A., McClelland G. A. Electrophoretic differences between sympatric ecotypes. Nature. 1975 Jul 31;256(5516):405–406. doi: 10.1038/256405a0. [DOI] [PubMed] [Google Scholar]


