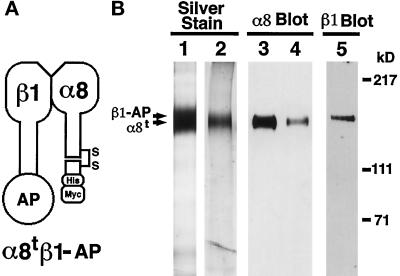
Figure 1.
Purification of soluble truncated integrin α8β1 heterodimer and α8 monomer. (A) Schematic representation of the soluble α8tβ1-AP heterodimer. Mature integrin α8 and β1 subunits consist of extracellular, transmembrane, and cytoplasmic domains. The truncated α8 (α8t) consists of the entire extracellular domain of mouse α8 cDNA with c-terminal (His)6 and c-myc epitope tags. The β1-AP chimera (β1-AP) consists of the entire extracellular domain of β1 fused in frame to human placental AP. α8tβ1-AP is a heterodimer of α8t and β1-AP. (B) The purified α8tβ1-AP heterodimer (lanes 1, 3, and 5) and α8t monomer (lanes 2 and 4) were separated by electrophoresis in 6% polyacrylamide gels in nonreducing conditions. The gels were either silver stained (lanes 1 and 2) or transferred to nitrocellulose membranes and probed with anti-α8 antiserum (lanes 3 and 4) or anti-β1 mAb 9EG7 (lane 5). In lane 1, both α8t and β1-AP bands are within the overexposed band shown. The purified α8t monomer (lane 2) was used as an antigen to prepare the rabbit polyclonal anti-mouse α8 antibody.