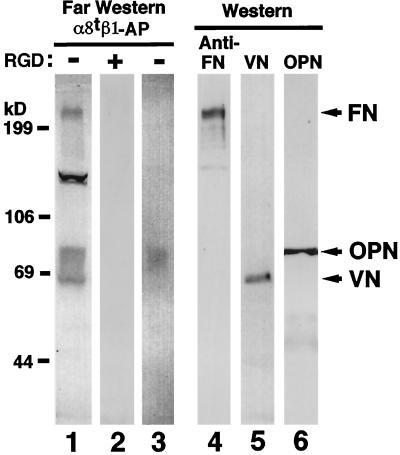
Figure 4.
RGD-dependent binding of α8tβ1-AP to proteins including OPN in mouse embryo extract. Fifty micrograms of protein per lane of the extract from E13.5 embryo (lanes 1, 2, and 4–6) and immunoprecipitates from E16.5 kidney extract with OPN antiserum pp69 (lane 3) were separated electrophoretically on a 7.5% polyacrylamide gel in reducing conditions and analyzed by far Western (lanes 1–3) and Western (lanes 4–6) blotting. For far Western blotting of the E13.5 extract, membrane strips were incubated with α8tβ1-AP in the absence (lane 1) or presence (lane 2) of 50 μg/ml GRGDSP peptide. For Western blotting, the membrane strips were probed with anti-FN IgG (lane 4), anti-VN IgG (lane 5), or anti-OPN mAb MPIIIB10 (lane 6). The integrin-AP chimera bound to protein bands of 240, 130, and 65–85 kDa in the extract (lane 1) and ∼80 kDa in the anti-OPN immunoprecipitates (lane 3). This 80-kDa band comigrates with the band detected by OPN Western blot (lane 6).