Abstract
The vaccinia virus growth factor (VGF) gene encodes a polypeptide with amino acid sequence homology to epidermal growth factor (EGF) and transforming growth factor alpha and is present twice, once at each end of the virus genome within the inverted terminal repetition. Recombination procedures were used to replace more than half of both VGF genes with a beta-galactosidase cassette which served as a color indicator for isolating an unconditionally viable VGF- mutant. The VGF- mutant genotype and phenotype were confirmed by Southern blot analysis and assays for functional growth factor. The plaque-forming efficiencies of VGF- and wild-type (WT) viruses were similar in a variety of cell types containing low or high densities of EGF receptors, suggesting a lack of a specific requirement for either VGF or the EGF receptor in the initiation of virus infection. The yield of VGF- virus was similar to that of WT virus in growing BS-C-1 and Swiss 3T3 cells, but lower in resting Swiss 3T3 cells. The greatest differences between VGF- and WT virus occurred in vivo: higher doses of VGF- virus than WT virus were required for intracranial lethality in mice and for production of skin lesions in rabbits. Thus, expression of the VGF gene is important to the virulence of vaccinia virus.
Full text
PDF
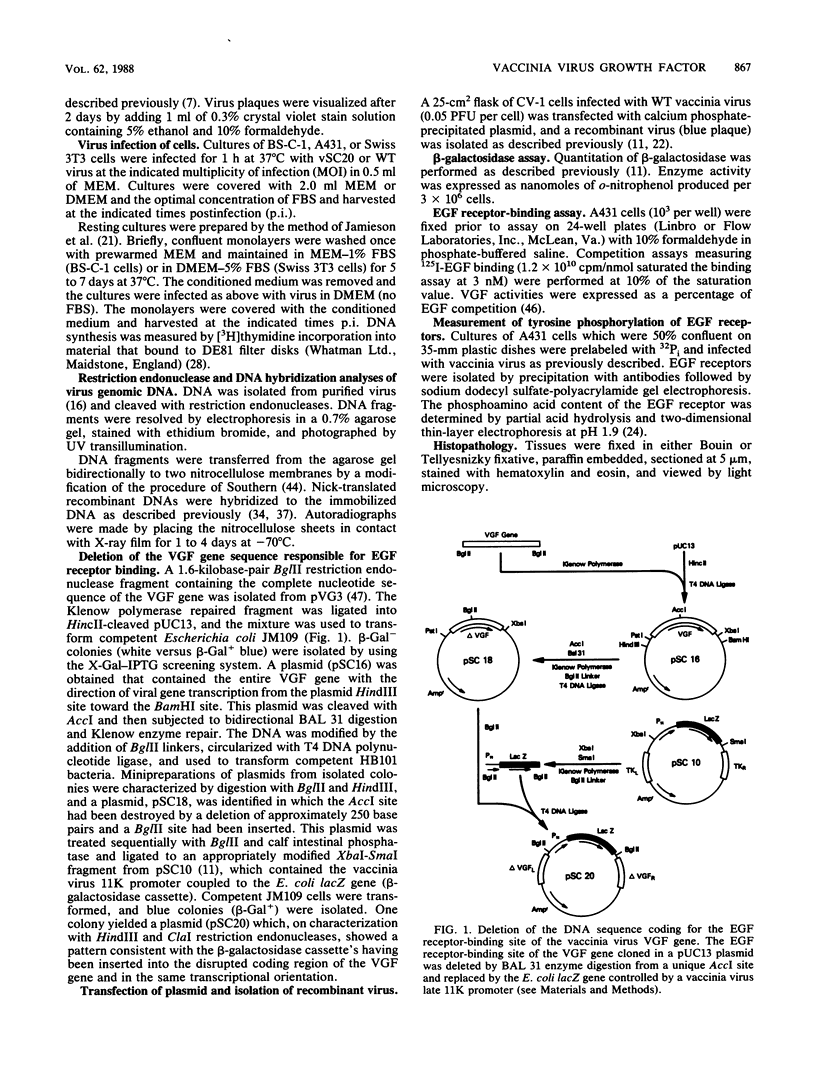
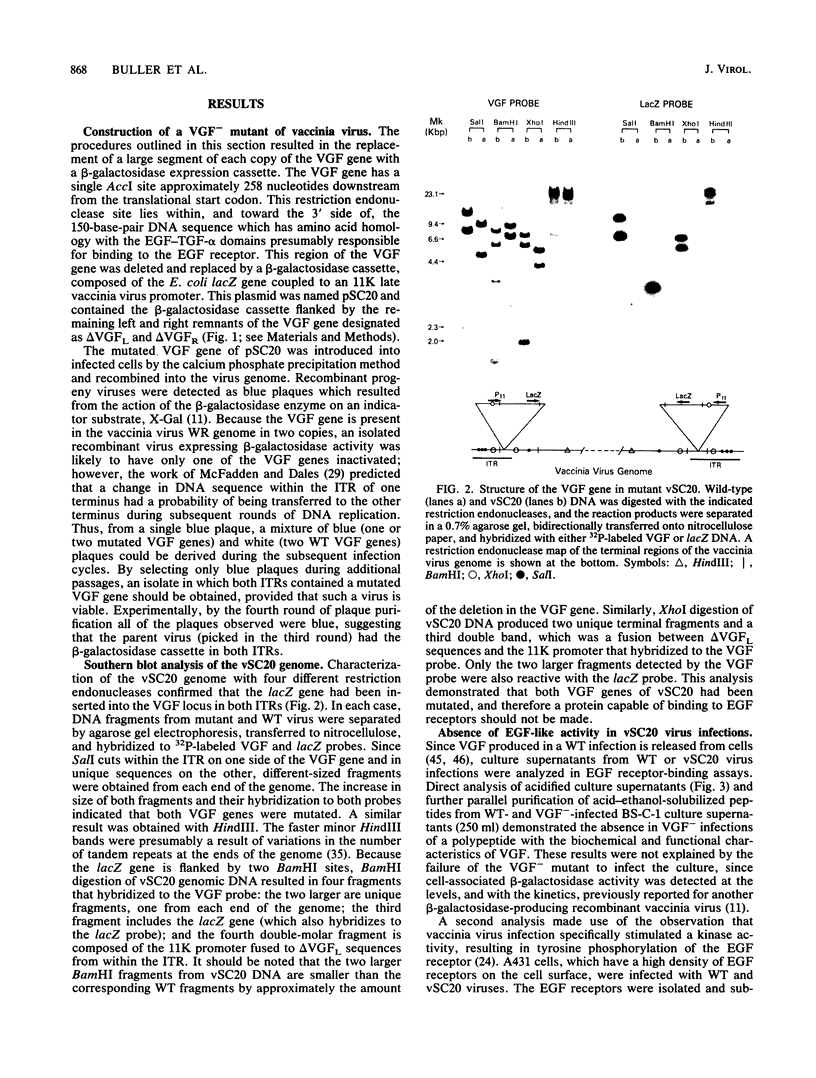



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomquist M. C., Hunt L. T., Barker W. C. Vaccinia virus 19-kilodalton protein: relationship to several mammalian proteins, including two growth factors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7363–7367. doi: 10.1073/pnas.81.23.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature. 1985 Feb 7;313(6002):491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Wallace G. D. Reexamination of the efficacy of vaccination against mousepox. Lab Anim Sci. 1985 Oct;35(5):473–476. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Epidermal growth factor: biology and receptor metabolism. J Cell Sci Suppl. 1985;3:1–9. doi: 10.1242/jcs.1985.supplement_3.1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Zendegui J. G. Epidermal growth factor, its receptor, and related proteins. Exp Cell Res. 1986 May;164(1):1–10. doi: 10.1016/0014-4827(86)90449-0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Binding of phorbol esters to high-affinity sites on murine fibroblastic cells elicits a mitogenic response. J Cell Physiol. 1982 Jul;112(1):42–50. doi: 10.1002/jcp.1041120108. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Marsh Y. V., Schreiber A. B., Newman S. R., Todaro G. J., Nestor J. J., Jr Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature. 1985 Dec 19;318(6047):663–665. doi: 10.1038/318663a0. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Armour R., Baldwin J. H., Brown K. D., Yeh Y. C. Density-dependent regulation of growth of BSC-1 cells in cell culture: control of growth by serum factors. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5046–5050. doi: 10.1073/pnas.74.11.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. S., Cooper J. A., Moss B., Twardzik D. R. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol Cell Biol. 1986 Jan;6(1):332–336. doi: 10.1128/mcb.6.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Leutz A., Schachner M. Cell type-specificity of epidermal growth factor (EGF) binding in primary cultures of early postnatal mouse cerebellum. Neurosci Lett. 1982 May 28;30(2):179–182. doi: 10.1016/0304-3940(82)90293-2. [DOI] [PubMed] [Google Scholar]
- Leutz A., Schachner M. Epidermal growth factor stimulates DNA-synthesis of astrocytes in primary cerebellar cultures. Cell Tissue Res. 1981;220(2):393–404. doi: 10.1007/BF00210517. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960 Feb;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: mirror-image deletions in vaccinia virus DNA. Cell. 1979 Sep;18(1):101–108. doi: 10.1016/0092-8674(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Howanitz J., van Gemert M., Miller M. Clofibrate-induced antidiuresis. J Clin Invest. 1973 Mar;52(3):535–542. doi: 10.1172/JCI107213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper J. A. Deletion of a 9,000-base-pair segment of the vaccinia virus genome that encodes nonessential polypeptides. J Virol. 1981 Nov;40(2):387–395. doi: 10.1128/jvi.40.2.387-395.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper N. Instability and reiteration of DNA sequences within the vaccinia virus genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1614–1618. doi: 10.1073/pnas.78.3.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J. A. Histopathogenesis of mousepox. II. Cutaneous infection. Br J Exp Pathol. 1962 Oct;43:462–468. [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H. Similarity between the vaccinia virus 19K early protein and epidermal growth factor. 1985 Feb 28-Mar 6Nature. 313(6005):801–803. doi: 10.1038/313801a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J. D., Kawamoto T., Le A. D., Mendelsohn J., Polikoff J., Sato G. H. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983 Dec;1(5):511–529. [PubMed] [Google Scholar]
- Simon J., Werner G. T. Vaccinia virus infection of the central nervous system in X-irradiated mice. Infect Immun. 1979 Sep;25(3):1035–1042. doi: 10.1128/iai.25.3.1035-1042.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Morrison R., de Vellis J., Herschman H. R. Epidermal growth factor binding and mitogenic activity on purified populations of cells from the central nervous system. J Neurosci Res. 1982;8(2-3):453–462. doi: 10.1002/jnr.490080233. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Rice A. P., Gullick W. J., Cheng D. J., Kerr I. M., Waterfield M. D. Purification and characterization of vaccinia virus growth factor. Cell. 1985 Aug;42(1):383–393. doi: 10.1016/s0092-8674(85)80133-1. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B. Density dependent proliferation of human glia cells stimulated by epidermal growth factor. Biochem Biophys Res Commun. 1976 Mar 22;69(2):304–310. doi: 10.1016/0006-291x(76)90522-2. [DOI] [PubMed] [Google Scholar]
- Wittek R., Barbosa E., Cooper J. A., Garon C. F., Chan H., Moss B. Inverted terminal repetition in vaccinia virus DNA encodes early mRNAs. Nature. 1980 May 1;285(5759):21–25. doi: 10.1038/285021a0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]