Table 2.
Iron-catalyzed carbomagnesiation of propargylic and homopropargylic alcohols.a
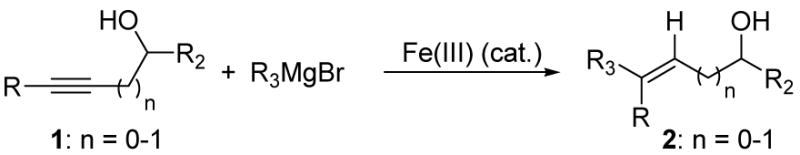 | |||
|---|---|---|---|
| entry | Conditions | Product | Yield (%)b |
| 1 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) |
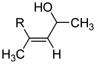
R = n-C10H21 |
75 |
| 2 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) | R = TBSO(CH2)4 | 80 |
| 3 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) | R = BnO(CH2)3 | 70 |
| 4 | Fe(acac)3 (0.20 equiv) dppe (0.20 equiv) |
|
78 |
| 5 | Fe(acac)3 (0.30 equiv) dppe (0.30 equiv) |
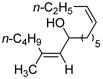
|
85 |
| 6 | Fe(acac)3 (0.15 equiv) |
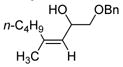
|
81 |
| 7 | Fe(acac)3 (0.30 equiv) |
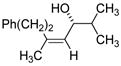
99% ee |
61 |
| 8 | Fe(ehx)3 (0.30 equiv) dppe (0.30 equiv) |
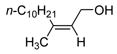
|
80 |
| 9c | Fe(acac)3 (0.20 equiv) |
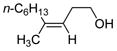
|
75 |
| 10c | Fe(acac)3 (0.20 equiv) |
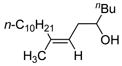
|
74 |
| 11 | Fe(acac)3 (0.50 equiv) CuBr (0.60 equiv) |
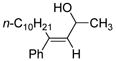
|
69 |
| 12c | Fe(acac)3 (0.40 equiv) |
|
63 |
| 13d | Fe(acac)3 (0.20 equiv) NMP (2.0 equiv) |
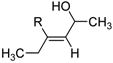
R=n-C10H21 |
70 |
| 14d | Fe(acac)3 (0.20 equiv) NMP (2.0 equiv) | R= TBSO(CH2)4 | 74 |
Reactions carried out in THF (0.1M in substrate) using 5.0 equivalents of RMgBr at 0 °C for 7h unless otherwise indicated.
Isolated yield.
In toluene at 23 °C.
NMP = N-methyl pyrrolidine.
