Abstract
We tested the hypotheses that arterial baroreflex (ABR) control over muscle sympathetic nerve activity (MSNA) in humans does not remain constant throughout a bout of leg cycling ranging in intensity from very mild to exhausting. ABR control over MSNA (burst incidence, burst strength and total MSNA) was evaluated by analysing the relationship between beat-to-beat spontaneous variations in diastolic arterial pressure (DAP) and MSNA in 15 healthy subjects at rest and during leg cycling in a seated position at five workloads: very mild (10 W), mild (82 ± 5.0 W), moderate (126 ± 10.2 W), heavy (156 ± 14.3 W), and exhausting (190 ± 21.2 W). The workload was incremented every 6 min. The linear relationships between DAP and MSNA variables were significantly shifted downward during very mild exercise, but then shifted progressively upward as exercise intensity increased. During heavy and exhausting exercise, moreover, the DAP–MSNA relationships were also significantly shifted rightward from the resting relationship. The sensitivity of ABR control over burst incidence and total MSNA was significantly lower during very mild exercise than during rest, and the sensitivity of the burst incidence control remained lower than the resting level at all higher exercise intensities. By contrast, the sensitivity of the total MSNA control recovered to the resting level during mild and moderate exercise, and was significantly increased during heavy and exhausting exercise (versus rest). We conclude that, in humans, ABR control over MSNA is not uniform throughout a leg cycling exercise protocol in which intensity was varied from very mild to exhausting. We suggest that this non-uniformity of ABR function is one of the mechanisms by which sympathetic and cardiovascular responses are matched to the exercise intensity.
During dynamic exercise, mean arterial pressure (MAP), heart rate (HR) and sympathetic nerve activity (SNA) all increase in response to a progressive increase in workload (Rowell et al. 1996). It has been suggested that these cardiovascular responses are mediated by central command (Rowell et al. 1996) as well as by feedback mechanisms via afferent nerves (group III and IV fibres) arising from the working skeletal muscles (Mitchell & Schmidt, 1983; Mitchell, 1990; Rowell & O'Leary, 1990), and are modulated by arterial and cardiopulmonary baroreflexes (Rowell & O'Leary, 1990; Rowell et al. 1996). However, the arterial baroreflex (ABR)-mediated regulation of SNA during dynamic exercise is not yet fully understood.
To the best of our knowledge, only three studies have examined ABR control of SNA during dynamic exercise in humans by using microneurographic recordings of muscle sympathetic nerve activity (MSNA) (Fadel et al. 2001; Keller et al. 2004; Ogoh et al. 2007). Fadel et al. (2001) reported that carotid baroreflex control of MSNA remains constant during moderate-intensity arm cycling, while Keller et al. (2004) reported it was also unchanged during mild-intensity one-legged kicking. Moreover, Ogoh et al. (2007) recently reported that ABR control of MSNA is progressively reset to higher blood pressures during the transition from rest to steady state moderate arm cycling with no change in reflex sensitivity. Thus all three of these studies suggest that ABR control of MSNA is well preserved during dynamic exercise up to moderate intensity, but ABR control of MSNA during dynamic exercise at intensities higher than moderate is unknown in humans.
In contrast to the findings of the aforementioned human studies, Miki et al. (2003) observed that ABR control over renal SNA is reset to higher blood pressures and higher SNA levels, with a significant increase in ABR sensitivity, during high intensity (∼70% of the maximum oxygen consumption) treadmill exercise in rats. In addition, significant increases in the sensitivity of ABR control over MSNA have been observed during isometric exercise in humans and, importantly, it was activation of the muscle metaboreflex that triggered the increase in sensitivity (Kamiya et al. 2001; Ichinose et al. 2004b, 2006b). It may well be that the intensities of the dynamic exercise employed in those earlier studies, in which ABR control of MSNA was unchanged, did not fully activate the muscle metaboreflex (Fadel et al. 2001; Keller et al. 2004; Ogoh et al. 2007), and that dynamic exercise at a workload high enough to activate the muscle metaboreflex would increase the sensitivity of ABR control of MSNA in humans. In addition, several other factors that reportedly influence ABR function, including central command, the muscle mechanoreflex, body temperature, and central venous pressure (CVP) (Iellamo et al. 1997; Potts & Mitchell, 1998; Gallagher et al. 2001; McIlveen et al. 2001; Cui et al. 2002; Ogoh et al. 2002; Kamiya et al. 2003; Charkoudian et al. 2004; Yamamoto et al. 2004), would also be progressively activated or altered as exercise intensity increased (Rowell et al. 1996). Moreover, the responses to dynamic exercise of the factors that affect ABR function would differ considerably from their responses to isometric exercise (Rowell, 1993; Rowell et al. 1996). Consequently, ABR function during dynamic exercise could be very different from that during isometric exercise.
It is recognized that cardiovascular and MSNA responses to dynamic exercise are affected by a variety of factors, including exercise mode, muscle mass and position during exercise (Bevegård et al. 1966; Stenberg et al. 1967; Victor & Seals, 1989; Ray et al. 1993; Saito et al. 1993, 1997). It is therefore possible that these factors also influence ABR control of MSNA during dynamic exercise. Previous studies investigating ABR control over MSNA employed dynamic exercise with a relatively small muscle mass (i.e. arm cycling or one-legged kicking) (Fadel et al. 2001; Keller et al. 2004; Ogoh et al. 2007). However, the ABR-mediated regulation of MSNA during dynamic leg exercise involving a large muscle mass (i.e. two-legged cycling in a seated posture), which is one of the most extensively used dynamic exercise models for physiological studies (Saltin & Hermansen., 1966; Schemidt & Brück. 1981; Saito et al. 1993, 1997, 1999; Callister & Seals. 1994; Gallagher et al. 2001; Nishiyasu et al. 2001; Kenny & Niedre. 2002; Hayashi et al. 2004), has never been examined and might be different from that observed during dynamic exercise with a small muscle mass. Our aim in the present study was to test the working hypotheses that, in humans, ABR control over MSNA will not remain constant throughout a leg cycling exercise protocol during which intensity is incrementally increased from very mild to exhausting.
Methods
Subjects
We studied 15 healthy volunteers (13 men and 2 women) with a mean age of 28 ± 2 year, mean body weight of 63.3 ± 2.8 kg and mean height of 171.0 ± 2.1 cm. The subjects were all non-smokers and none was taking any medication. They also did not participate in any regular athletic training, but were relatively active and in good health. The study, which was carried out in accordance with the Declaration of Helsinki, was approved by the Human Subjects Committee of the University of Tsukuba, and each subject gave informed written consent.
Procedures
Each subject performed an incremental leg cycling exercise on an electrically braked ergometer (Aerobike 800, Combi, Tokyo, Japan) in a semirecumbent position (i.e. the upper body was leaning back about 25 degrees). The exercise had five workload levels selected as very mild (10 W), mild (82 ± 5.0 W), moderate (126 ± 10.2 W), heavy (156 ± 14.3 W) and exhausting (190 ± 21.2 W). The workload was incremented every 6 min, and the exercise at the highest intensity was continued until volitional exhaustion (5–7 min). Five subjects accomplished the entire incremental exercise protocol. With 10 subjects, the protocol was terminated before completion because the MSNA signal was no longer sufficiently clear for analysis (the exercise protocol was terminated at the level of mild exercise for three subjects, moderate for four subjects, heavy for one subject, and exhausting for two subjects). Data for these 10 subjects were therefore obtained until exercise intensity reached one level below the termination level. Control (resting) data were acquired for 5 min before the start of the exercise. We used data obtained during the last 3 min of each level of exercise for analysis. From the 10 min recording made during recovery after exhausting exercise, we used the 3rd to the 5th minute as the first phase of recovery (Rec1) and the 8th to the 10th minute as the second phase of recovery (Rec2).
Measurements
HR was monitored via a three-lead electrocardiogram (ECG). Beat-to-beat changes in blood pressure were monitored by finger photoplethysmography (Finapres 2300; Ohmeda, Englewood, CO, USA) using a cuff placed around the middle finger; the forearm and hand were supported so that the cuff was aligned at heart level. The Finapres measurements were confirmed by mercury sphygmomanometer blood pressure measurements prior to data collection.
Multiunit muscle sympathetic nerve discharges were recorded using the microneurographic technique. A tungsten microelectrode with a shaft diameter of 0.1 mm and an impedance of 1–5 MΩ was inserted manually by an experimenter into the median nerve at the cubital fossa and then adjusted until MSNA was encountered (Saito et al. 1993, 1997). The criteria for MSNA were spontaneous burst discharges that were synchronized with the heart beat and enhanced by Valsalva's manoeuvre or apnoea, but were unaffected by cutaneous touch or arousal stimuli (Delius et al. 1972; Vallbo et al. 1979; Saito et al. 1993). The neurogram was fed to a differential amplifier and amplified 100 000 times through a band-pass filter (500–3000 Hz), then full-wave rectified and integrated using a capacitance-integrated circuit with a time constant of 0.1 s. The analog signals representing the ECG, blood-pressure waveforms, and the mean voltage neurogram (see below) were continuously recorded on an FM magnetic-tape data-recorder (MR-30; TEAC, Tokyo, Japan). The data were also digitized at a sampling frequency of 400 Hz through an analog-to-digital converter (Maclab/8e; ADInstruments, Castle Hill, Australia), then fed into a personal computer (Powerbook 1400C; Apple, Tokyo, Japan). In addition, individual ratings of perceived exertion (RPE; based on the 6–20 Borg scale) were obtained at the 3rd min and the end of each exercise level, after which the two RPE values obtained in each level were averaged to provide one representative value (Borg, 1982).
Data analysis
Beat-to-beat heart rate was calculated from the R-R intervals on the ECG. Beat-to-beat systolic and diastolic arterial pressures (SAP and DAP, respectively) were obtained from the arterial pressure waveform. MAP was calculated using the equation MAP = DAP + (SAP – DAP)/3.
During the 5 min rest period prior to exercise, MSNA bursts were identified by inspection of the mean voltage neurogram, after which the voltage levels during the periods between bursts were averaged, and this level was taken as zero. The largest burst amplitude occurring during the resting period was assigned a value of 1000, after which MSNA data were normalized with respect to this standard in each subject. The amount of sympathetic nerve activity under each condition was expressed as burst frequency (bursts min−1) and burst incidence (burst/100 heartbeats). Burst strength, obtained from the mean area of the MSNA bursts recorded under each condition, was expressed as mean burst strength (arbitrary units). Total MSNA was taken as the product of mean burst strength and burst frequency.
Assessment of ABR control over burst incidence, burst strength and total MSNA using spontaneous beat-to-beat fluctuations in both blood pressure and MSNA has been described in detail elsewhere (Ichinose et al. 2004b, 2006b). Briefly, we investigated the ABR control over MSNA parameters during the resting period, at each exercise level, and during recovery as follows. First, taking into account the latency between the R wave of the ECG and the sympathetic burst (Fagius & Wallin., 1980), the DAP for each individual heartbeat was related to the corresponding MSNA data. We used DAP in this analysis because changes in MSNA correlate closely with changes in DAP, but not with changes in SAP (Sundlof & Wallin. 1978b). Second, all DAP values measured under each condition were grouped into 1 mmHg bins. In each group, diastoles were inspected to determine whether or not they were associated with an MSNA burst, after which the percentage of diastoles associated with each MSNA burst (burst incidence/beat) was calculated. Third, we used signal averaging to determine the burst strength and total MSNA activity for each diastolic-pressure bin (Halliwill, 2000). Briefly, the MSNA signals were averaged over a period corresponding to the length of the heartbeat, taking into account the presumed latency from the R wave of the ECG, after which the area under the averaged MSNA signal was calculated. To calculate the burst strength related to each diastolic-pressure bin (burst strength/beat), those MSNA signals associated with a burst were selected and averaged, which enabled us to calculate the area of the averaged MSNA signal using the above-mentioned technique. The total MSNA related to each diastole-pressure bin (total activity/beat) was calculated as the area of the averaged MSNA signal created from all the MSNA signals in each bin, whether or not they were associated with an MSNA burst. Therefore, if a cardiac cycle did not have a burst associated with it, an MSNA signal without a burst was included in the averaging of the total MSNA for that bin. Finally, the calculated burst incidence, burst strength and total MSNA obtained for each diastolic-pressure bin was plotted against the corresponding DAP, and linear regression analysis was performed for each diagram. At the higher blood pressures tested, MSNA was often completely inhibited (i.e. burst incidence went to zero at the high DAPs). Within this range, MSNA did not change despite changes in DAP (i.e. burst incidence was always zero); however, below this high DAP range, MSNA is negatively correlated with DAP. When we found the blood pressure to be in the high range, the highest DAP included in the regression analysis was selected from the first three DAPs in the high blood pressure range, so as to obtain the largest correlation coefficient for the linear regression line. For example, if the correlation coefficient was largest when the first two DAPs in the high blood pressure range were included in the regression analysis (as compared to when the first DAP or first three DAPs were included), the second DAP in the high blood pressure range was selected to be the highest DAP on the linear regression line. We applied this criterion to eliminate data in the high blood pressure range while maintaining consistency among conditions, and to avoid introducing subjectivity into the analysis. The linear regression analysis was performed with and without weighting the data with respect to the numbers of cardiac cycles in the bins (the technique for weighting the data is described in the Methods and Discussion of earlier reports by Kienbaum et al. 2001 and Keller et al. 2006). The number of DAP bins used for regression analysis during rest, at each workload and during the recovery periods were not different. We took the slope of each regression line as indicating the ABR sensitivity in the control over each variable. The points corresponding to the average DAP on the regression lines relating burst incidence or total MSNA to DAP were taken as the prevailing points for a given relationship and as an index of the MSNA corresponding to the ABR operating pressure. Because of the weak relationship between burst strength and DAP (described in detail in the Results and Discussion), we did not calculate the prevailing point for the regression line relating burst strength to DAP.
Statistical analysis
Data are presented as means ± s.e.m. For physiological responses (arterial blood pressure, HR and MSNA) and for the slope and prevailing point of the linear relationships between DAP and burst incidence and DAP and total MSNA, comparisons among rest and each exercise intensity were made using a one-way repeated measures analysis of variance with the data obtained from five subjects who completed all the stages. To compare the slopes of the linear relationships between DAP and burst strength, we used a one-way non-repeated analysis of variance because each setting had an independent sample (i.e. as a result of the weak relationship between DAP and burst strength, the subjects differed for each setting). Fisher's post hoc test was used to assess differences between group means. The characteristics of the relationships between MSNA (burst incidence, burst strength and total MSNA) and DAP were determined by least-squares linear-regression analysis. Values of P < 0.05 were considered significant. The data presented in Tables 1, 2 and 4 are group averages from all subjects, including the subjects who could not accomplish the entire exercise protocol. The data presented in Table 3 are group averages from subjects for whom there was a significant linear relationship between DAP and burst strength. The number of subjects in each condition is shown in the tables.
Table 1.
Arterial blood pressure, heart rate and MSNA during the resting period, at each level of exercise, and during the recovery periods
| Rest | Very mild | Mild | Moderate | Heavy | Exhausting | Rec1 | Rec2 | |
|---|---|---|---|---|---|---|---|---|
| SAP (mmHg) | 136 ± 3.1 | 156 ± 3.2* | 177 ± 4.7* | 187 ± 5.6* | 195 ± 7.3*† | 199 ± 10.4*† | 123 ± 5.6† | 121 ± 7.7† |
| DAP (mmHg) | 66 ± 1.9 | 65 ± 2.0 | 66 ± 2.8 | 69 ± 1.8 | 73 ± 2.3* | 75 ± 2.8*† | 58 ± 2.5*† | 64 ± 2.5 |
| MAP (mmHg) | 89 ± 1.8 | 95 ± 1.5* | 103 ± 2.7* | 109 ± 2.4* | 114 ± 3.2*† | 116 ± 4.4*† | 79 ± 3.6*† | 83 ± 4.3† |
| HR (beats min−1) | 74 ± 3.1 | 85 ± 3.3* | 108 ± 3.4* | 131 ± 5.6* | 160 ± 5.9*† | 179 ± 7.1*† | 129 ± 7.6*† | 114 ± 7.1*† |
| MSNA burst frequency (bursts min−1) | 26.3 ± 1.8 | 19.1 ± 1.8* | 24.6 ± 2.3 | 33.7 ± 3.6* | 46.2 ± 4.7*† | 66.0 ± 5.2*† | 55.6 ± 1.5*† | 54.1 ± 3.4*† |
| MSNA burst incidence (bursts 100 HR−1) | 35.3 ± 2.5 | 22.5 ± 2.0* | 22.8 ± 2.0* | 25.7 ± 2.8* | 29.3 ± 3.5 | 37.3 ± 3.0† | 43.7 ± 3.1*† | 48.4 ± 4.3*† |
| Mean burst strength (AU) | 117.9 ± 5.4 | 101.0 ± 4.4* | 115.8 ± 7.4 | 137.2 ± 9.5* | 172.0 ± 12.3*† | 220.8 ± 16.6*† | 180.9 ± 11.6*† | 169.6 ± 12.6*† |
| Total MSNA (AU) | 3128 ± 289 | 1877 ± 167* | 2882 ± 365 | 4671 ± 581* | 8186 ± 1201*† | 14792 ± 1817*† | 10092 ± 799*† | 9291 ± 1053*† |
| RPE | — | 8.1 ± 0.4 | 12.0 ± 0.3 | 14.3 ± 0.4 | 17.0 ± 0.3 | 19.2 ± 0.2 | — | — |
| Work load (watt) | — | 10 | 82 ± 5.0 | 126 ± 10.2 | 156 ± 14.3 | 190 ± 21.2 | — | — |
| Number of subjects | 15 | 15 | 12 | 8 | 7 | 5 | 5 | 5 |
SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity; AU, arbitrary units; Rec1, 3–5 min after the end of the exhausting exercise; Rec2, 8–10 min after the end of the exhausting exercise. Total MSNA was taken as the product of mean burst strength and burst frequency. Statistical indicators depict responses between the indicated conditions for the five subjects who completed all stages.
P < 0.05 versus Rest;
P < 0.05 versus Moderate. Numerical data are group averages from all subjects (including the subjects who could not accomplish the entire exercise protocol) ± s.e.m.
Table 2.
Derived variables describing the arterial baroreflex control over MSNA burst incidence
| Rest | Very mild | Mild | Moderate | Heavy | Exhausting | Rec1 | Rec2 | |
|---|---|---|---|---|---|---|---|---|
| Results of the linear regression analysis performed with weighting of the data | ||||||||
| Slope of incidence line (bursts 100 HR−1 mmHg−1) | −4.56 ± 0.24 | −3.22 ± 0.23* | −2.86 ± 0.18* | −2.90 ± 0.39* | −3.55 ± 0.17* | −3.06 ± 0.38* | −4.04 ± 0.31† | −4.42 ± 0.34† |
| Correlation coefficient | −0.80 ± 0.01 | −0.73 ± 0.02 | −0.79 ± 0.02 | −0.73 ± 0.02 | −0.66 ± 0.03 | −0.72 ± 0.03 | −0.82 ± 0.03 | −0.78 ± 0.01 |
| Prevailing point (bursts 100 HR−1) | 33.2 ± 2.74*† | 19.8 ± 1.75 | 20.5 ± 2.27* | 26.0 ± 2.81* | 29.4 ± 3.52* | 33.9 ± 3.09 | 43.2 ± 2.72† | 45.5 ± 3.21*† |
| Results of the linear regression analysis performed without weighting of the data | ||||||||
| Slope of incidence line (bursts 100 HR−1 mmHg−1) | −4.90 ± 0.28 | −3.51 ± 0.26* | −3.06 ± 0.18* | −3.02 ± 0.43* | −3.78 ± 0.17* | −3.26 ± 0.40* | −4.27 ± 0.35† | −4.68 ± 0.37† |
| Correlation coefficient | −0.88 ± 0.02 | −0.81 ± 0.02 | −0.84 ± 0.02 | −0.80 ± 0.03 | −0.70 ± 0.04 | −0.79 ± 0.03 | −0.89 ± 0.03 | −0.86 ± 0.01 |
| Prevailing point (bursts 100 HR−1) | 34.9 ± 2.87 | 20.7 ± 1.82* | 21.6 ± 2.38* | 27.4 ± 2.96* | 31.1 ± 3.81 | 35.5 ± 3.25† | 45.6 ± 2.87*† | 47.9 ± 3.38*† |
Results of the linear regression analysis performed with and without weighting of the data are shown. Prevailing point, point on the regression line corresponding to the average diastolic blood pressure. Statistical indicators depict responses between the indicated conditions for the five subjects who completed all stages.
P < 0.05 versus Rest;
P < 0.05 versus Moderate. Numerical data are group averages from all subjects (including the subjects who could not accomplish the entire exercise protocol) ± s.e.m. Abbreviations and the number of subjects in each condition are the same as in Table 1.
Table 4.
Derived variables describing the arterial baroreflex control over total MSNA
| Rest | Very mild | Mild | Moderate | Heavy | Exhausting | Rec1 | Rec2 | |
|---|---|---|---|---|---|---|---|---|
| Results of the linear regression analysis performed with weighting of the data | ||||||||
| Slope of total MSNA line (units beat−1 mmHg−1) | −4.97 ± 0.33 | −3.80 ± 0.22* | −4.18 ± 0.39 | −4.96 ± 0.49 | −7.63 ± 0.44*† | −9.01 ± 0.58*† | −6.65 ± 0.48*† | −6.25 ± 0.45*† |
| Correlation coefficient | −0.77 ± 0.02 | −0.76 ± 0.02 | −0.74 ± 0.02 | −0.75 ± 0.03 | −0.71 ± 0.06 | −0.79 ± 0.02 | −0.76 ± 0.06 | −0.80 ± 0.04 |
| Prevailing point (units beat−1) | 52.4 ± 3.68 | 34.8 ± 2.63* | 45.3 ± 5.48 | 65.2 ± 4.49* | 83.8 ± 2.67*† | 124.9 ± 11.74*† | 100.2 ± 8.94*† | 96.6 ± 8.83*† |
| Results of the linear regression analysis performed without weighting of the data | ||||||||
| Slope of total MSNA line (units beat−1 mmHg−1) | −5.22 ± 0.35 | −4.14 ± 0.24* | −4.36 ± 0.40 | −5.17 ± 0.51 | −8.02 ± 0.46*† | −9.48 ± 0.60*† | −6.98 ± 0.51*† | −6.56 ± 0.48*† |
| Correlation coefficient | −0.84 ± 0.02 | −0.83 ± 0.02 | −0.83 ± 0.03 | −0.82 ± 0.04 | −0.77 ± 0.07 | −0.88 ± 0.03 | −0.84 ± 0.07 | −0.88 ± 0.04 |
| Prevailing point (units beat−1) | 55.9 ± 3.94 | 38.0 ± 2.83* | 48.0 ± 5.80 | 68.5 ± 4.76* | 89.0 ± 2.83*† | 130.8 ± 12.70*† | 105.5 ± 9.82*† | 102.98 ± 9.41*† |
Results of the linear regression analysis performed with and without weighting of the data are shown. Prevailing point, point on the regression line corresponding to the average diastolic blood pressure. Statistical indicators depict responses between the indicated conditions for the five subjects who completed all stages.
P < 0.05 versus Rest;
P < 0.05 versus Moderate. Numerical data are group averages from all subjects (including the subjects who could not accomplish the entire exercise protocol) ± s.e.m. Abbreviations and the number of subjects in each condition are same as in Table 1.
Table 3.
Derived variables describing the arterial baroreflex control over MSNA burst strength
| Rest | Very mild | Mild | Moderate | Heavy | Exhausting | Rec1 | Rec2 | |
|---|---|---|---|---|---|---|---|---|
| Results of the linear regression analysis performed with weighting of the data | ||||||||
| Slope of strength line (units beat−1 mmHg−1) | −3.31 ± 0.51 | −2.91 ± 0.63 | −3.01 ± 1.02 | −3.44 ± 0.73 | −4.28 ± 0.72 | −4.96 ± 0.53 | −3.65 ± 0.16 | −3.34 ± 0.09 |
| Correlation coefficient | −0.47 ± 0.05 | −0.40 ± 0.07 | −0.36 ± 0.11 | −0.53 ± 0.08 | −0.50 ± 0.07 | −0.58 ± 0.05 | −0.58 ± 0.04 | −0.55 ± 0.04 |
| Results of the linear regression analysis performed without weighting of the data | ||||||||
| Slope of strength line (units beat−1 mmHg−1) | −3.50 ± 0.55 | −3.16 ± 0.70 | −3.21 ± 1.09 | −3.58 ± 0.75 | −4.49 ± 0.75 | −5.21 ± 0.55 | −3.83 ± 0.17 | −3.49 ± 0.09 |
| Correlation coefficient | −0.51 ± 0.06 | −0.42 ± 0.08 | −0.38 ± 0.12 | −0.56 ± 0.09 | −0.53 ± 0.07 | −0.62 ± 0.05 | −0.63 ± 0.04 | −0.59 ± 0.04 |
| Number of subjects | 9 | 7 | 6 | 5 | 4 | 4 | 4 | 4 |
Results of the linear regression analysis performed with and without weighting of the data are shown. Comparisons among rest and the different exercise intensities were made using a one-way non-repeated analysis of variance with the data obtained from subjects for whom there was a significant linear relationship between DAP and burst strength. The data presented are group averages from subjects for whom there was a significant linear relationship between DAP and burst strength ± s.e.m. Abbreviations are same as in Table 1.
Results
Basal data
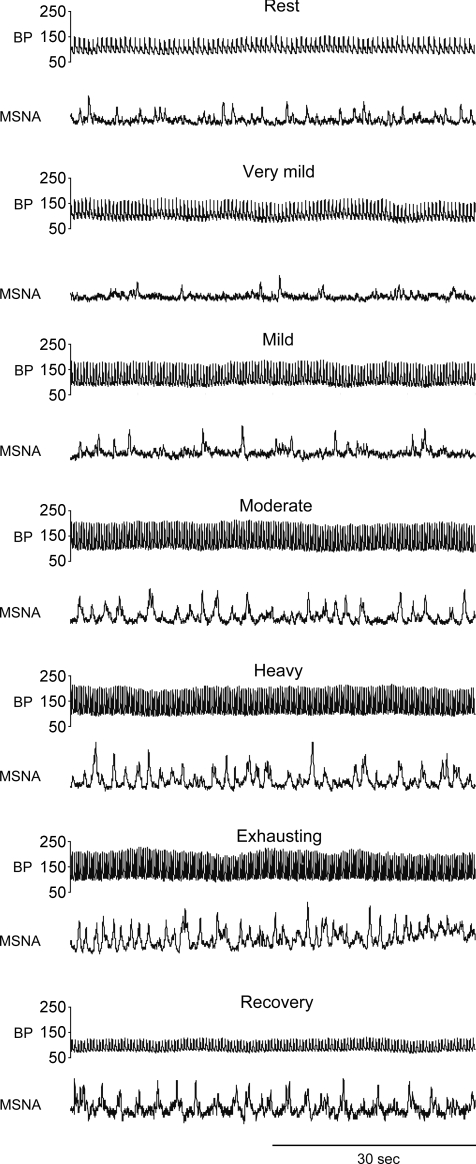
Raw recordings of arterial blood pressure and MSNA during the resting period, at each exercise level, and during the recovery period (Rec1) are shown in Fig. 1. Table 1 shows the group values obtained for arterial blood pressure, HR and MSNA during the indicated periods, as well as the RPE and workload for each level of exercise. During this incremental exercise, SAP, MAP and HR all increased progressively. Very mild to moderate exercise did not change DAP, though DAP was higher during heavy and exhausting exercise than during resting period. During very mild exercise, all of the MSNA variables were significantly lower than the resting values. Thereafter, burst frequency, burst strength and total MSNA increased progressively as exercise intensity increased, and during moderate exercise, these MSNA variables were significantly higher than the resting values. Meanwhile, burst incidence during mild and moderate exercise was lower than that at rest, though it tended to increase as exercise intensity increased, and reached the resting level during heavy and exhausting exercise. During recovery, SAP returned to the resting level, while DAP and MAP declined to a level below the resting level during Rec1 but recovered to resting level by Rec2. HR and all MSNA variables were higher than the resting values during both recovery periods. RPE gradually increased as exercise intensity increased.
Figure 1. Raw recordings of arterial blood pressure (BP) and MSNA.
Raw recordings of arterial blood pressure and MSNA during the resting period, very mild, mild, moderate, heavy and exhausting exercise and recovery period in a representative subject
ABR control over burst incidence, burst strength and total MSNA
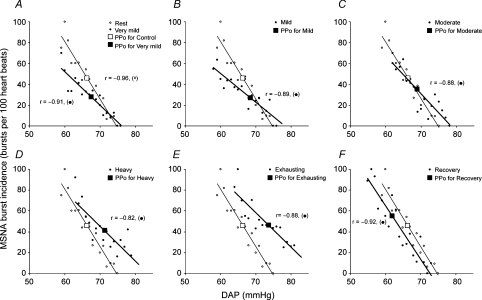
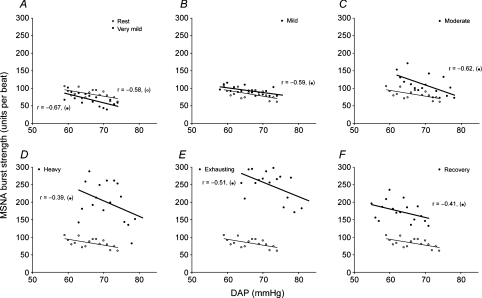
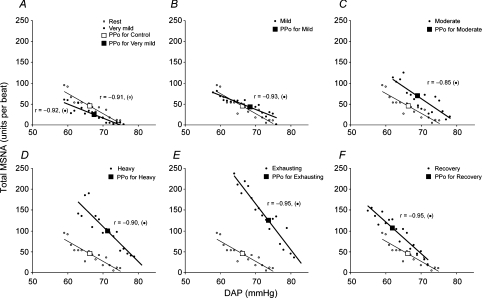
The linear regression lines relating burst incidence, burst strength and total MSNA to DAP during the resting period, at each exercise level, and during Rec1 in a representative subject are shown in Figs 2, 3 and 4, respectively. The derived variables describing ABR control over the three MSNA variables are presented for the group in Tables 2, 3 and 4, respectively. We reached the same conclusions whether or not the linear regression analysis was performed with or without weighting of the data. All of the subjects showed significant negative correlations between burst incidence or total MSNA and DAP under all conditions (Tables 2 and 4), but they did not all show a significant negative correlation between burst strength and DAP. The numbers of subjects who exhibited significant negative correlations between burst strength and DAP during the resting period, at each exercise level, and during the recovery periods were 9 (of 15), 7 (of 15), 6 (of 12), 5 (of 8), 4 (of 7), 4 (of 5), 4 (of 5) and 4 (of 5), respectively, and even among those who did show significant relationships, the correlation coefficients were smaller than those relating burst incidence or total MSNA to DAP (Table 3).
Figure 2. Linear relationships between MSNA burst incidence and DAP.
Linear relationships between MSNA burst incidence and DAP during the resting period (A–F, ○, thin lines); very mild (A, •, thick line), mild (B, •, thick line), moderate (C, •, thick line), heavy (D, •, heavy line) and exhaustive exercise (E, •, heavy line); and the recovery period (F, •, thick line) in a representative subject. The larger symbols show the prevailing points (PPo) at the indicated times during the experimental protocol. The regression lines shown in the figure were determined by linear regression analysis performed without weighting of the data.
Figure 3. Linear relationships between MSNA burst strength and DAP.
Linear relationships between MSNA burst strength and DAP during the resting period (A–F, ○, thin lines); very mild (A, •, thick line), mild (B, •, thick line), moderate (C, •, thick line), heavy (D, •, thick line) and exhaustive exercise (E, •, thick line); and the recovery period (F, •, thick line) in a representative subject. The regression lines shown in the figure were determined by linear regression analysis performed without weighting of the data.
Figure 4. Linear relationships between total MSNA and DAP in a representative subject.
The conventions are the same as in Fig. 2. The regression lines shown in the figure were determined by linear regression analysis performed without weighting of the data.
During very mild exercise, the linear relationships between the three MSNA variables and DAP, as well as the prevailing points, were shifted downward from the resting relationship (Tables 2, 3 and 4 and Figs 2A, 3A and 4A). The linear relationship between burst incidence and DAP and its prevailing point remained lower than the resting level during mild and moderate exercise, but it recovered to the resting level during heavy and exhausting exercise (Table 2, Fig. 2). At the same time, the linear relationships between burst strength and DAP and between total MSNA and DAP, and its prevailing point, all recovered to the resting level during mild exercise. Thereafter, they were all shifted progressively upward as exercise intensity increased (Tables 3 and 4 and Figs 3 and 4; the gradual upward shift in the relationship between burst strength and DAP was evidenced by progressive increases in mean burst strength). Also during heavy and exhausting exercise, the linear relationships between all three MSNA variables and DAP and the prevailing points were shifted to blood pressure levels that were higher than the resting pressures. The slopes of the burst incidence and total MSNA lines were smaller during very mild exercise than during the resting period (Tables 2 and 4 and Figs 2 and 4), and the slope of the burst incidence line remained lower than the resting slope at all higher exercise intensities (Table 2, Fig. 2). On the other hand, the slope of the burst strength line did not change significantly under any condition (Table 3, Fig. 3), but the slope of the total MSNA line recovered to the resting level during mild and moderate exercise, and was elevated during heavy and exhausting exercise (Table 4, Fig. 4).
During the recovery periods, the prevailing point of the burst incidence line shifted upward from the exhausting exercise level and was significantly higher than the resting level. By contrast, the linear relationships between burst strength and DAP and between total MSNA and DAP, and its prevailing point, were shifted downward from the exhausting exercise level, but remained significantly higher than the resting levels, and were comparable to, or even higher than, the heavy exercise level. During Rec1, the linear relationships between all MSNA variables and DAP and the prevailing points were shifted back to a pressure lower than under the resting condition, but had returned to the resting pressure during Rec2. The slope of the burst incidence line recovered to the resting value during the recovery periods. The slope of the total MSNA line declined from that seen during the exhausting exercise, but remained significantly greater than the resting slope.
Discussion
To our knowledge, this is the first study of ABR control over MSNA during an exercise protocol in which intensity (workload) was incrementally increased from very mild to exhausting in humans. The major finding of this investigation is that ABR control over MSNA is modulated during incremental dynamic exercise. Very mild exercise shifted the DAP–MSNA relationships to lower MSNA levels and also reduced ABR sensitivity. Incremental increases in exercise workload from mild to exhausting caused a gradual upward shift in the DAP–MSNA relationships. During moderate exercise, the sensitivity of the total MSNA control recovered to the resting level, and during heavy and exhausting exercise, there was a rightward and upward shift in the DAP–MSNA relations with a significant increase in the sensitivity of the control over total MSNA. Our findings during mild and moderate exercise are consistent with earlier studies showing that ABR sensitivity is unchanged during moderate-intensity arm cycling (Fadel et al. 2001; Ogoh et al. 2007) and mild-intensity one-legged kicking (Keller et al. 2004). Collectively, these results suggest that the sensitivity of ABR control over MSNA during mild to moderate dynamic exercise remains at the resting level, regardless of exercise mode. In addition, the modulation of ABR function we observed during heavy and exhausting exercise is in good agreement with the earlier report that in rats high intensity treadmill exercise resets the ABR control over renal SNA to higher blood pressures and higher SNA levels, with a significant increase in the ABR sensitivity (Miki et al. 2003). Bearing in mind those earlier studies and our present results, we suggest that ABR control over SNA is not uniform during dynamic exercise at different intensities.
Data on vascular sympathetic nerve activity during dynamic leg exercise using a large muscle mass (e.g. two-legged cycling) in humans are quite limited (Saito & Mano, 1991; Saito et al. 1993, 1997, 1999; Callister et al. 1994). Saito et al. (1993, 1999) investigated the MSNA response to leg cycling that was incrementally increased in intensity from very mild to exhausting. Those authors reported that MSNA burst frequency was significantly suppressed during low-level exercise (< 20% of maximal oxygen uptake (V̇O2,max)), but then increased as the exercise intensified and showed significantly higher burst frequency during moderate exercise (> 40%V̇O2,max than at rest. During exhausting exercise, burst frequency was increased by ∼250% over the resting level (Saito et al. 1999). The burst frequency responses observed in the present study are in good agreement with those earlier results. We observed a 267 ± 34% (n = 5) increase in burst frequency over the resting level during exhausting exercise. We also quantified burst strength and calculated total MSNA, which was advantageous because total MSNA is dependent on both burst frequency and burst strength and is therefore a better index for quantifying the level of MSNA than is either of those variables separately (Ray et al. 1993; Ichinose et al. 2004b, 2006b; Keller et al. 2006; Ogoh et al. 2007). During incremental cycling, mean burst strength changed in a manner similar to burst frequency, though its rate of change was smaller than that for burst frequency. During exhausting exercise, the average mean burst strength was increased 227 ± 23% (n = 5) over the resting level, while total MSNA was increased 617 ± 106% (n = 5) over the resting level. In addition, total MSNA increased 798 ± 77% from the lowest values observed during very-mild exercise. These results suggest that during the course of an incremental leg cycling exercise, MSNA will vary widely with exercise intensity, and vasomotor sympathetic activity will increase about eight-fold as exercise intensity is increased from very-mild to exhausting.
We do not know for certain the mechanism(s) responsible for the modulation of ABR control over MSNA seen during incremental dynamic exercise, though it is likely that these modulations reflect the interactions between ABR and other systems contributing to the regulation of SNA. Indeed, ABR function is reportedly influenced by central command as well as several peripheral neural inputs (Rowell & O'Leary, 1990; Iellamo et al. 1997; Potts & Mitchell, 1998; Cui et al. 2001, 2002; Gallagher et al. 2001; Kamiya et al. 2001, 2003; McIlveen et al. 2001; Ichinose et al. 2002, 2004b, 2006b; Ogoh et al. 2002; Charkoudian et al. 2004; Yamamoto et al. 2004), and these factors would be gradually activated or altered with the increasing workload during the dynamic exercise (Rowell et al. 1996). The inhibition of MSNA seen during very-mild leg cycling is thought to reflect the loading of both the cardiopulmonary and arterial baroreceptors due to relocation of blood which pooled in legs to the cardiopulmonary area and arterial side of the circulation secondary to the muscle pumping action in the legs (Ray et al. 1993; Saito et al. 1993, 1997, 1999). Furthermore, Charkoudian et al. (2004) demonstrated that increasing CVP via head-down tilt or saline infusion significantly attenuates ABR sensitivity in the control of MSNA. An increase in CVP and the resultant loading of both cardiopulmonary and arterial baroreceptors could therefore have contributed to the downward shift in the DAP–MSNA relations and the significant reduction in the sensitivity of ABR control over MSNA during very-mild exercise. In addition, it was previously shown that ABR control of burst incidence is preserved during isometric exercise (Ichinose et al. 2006b) and during mild arm cycling (Ogoh et al. 2007), whereas we found a reduction in the sensitivity during leg cycling up to the exhausting intensity. An increase in CVP during leg cycling might also account for the reduced sensitivity of burst incidence control, since isometric exercise and upper arm dynamic exercise would cause less important changes in CVP than dynamic leg exercise in a seated posture (Rowell, 1993).
Increasing afferent neural input from active skeletal muscles reflecting the progressive rise in workload also might be involved in the modulation of ABR control over MSNA. The results of earlier studies suggest that activation of the muscle metaboreflex could account for the upward and rightward shift of the DAP–MSNA relationships, as well as for the increase in the sensitivity of ABR control of MSNA observed during isometric exercise (Kamiya et al. 2001; Ichinose et al. 2006b). Although the level of dynamic exercise that activates the muscle metaboreflex in humans is not known, earlier studies using dogs suggest that it is not activated by mild exercise (treadmill running at 3.2–6.4 km h−1 with 0% grade), but it becomes tonically active at exercise intensities higher than moderate (6.4 km h−1 with 10% grade or 9.6 km h−1 with 0% grade) (Wyss et al. 1983; O'Leary & Sheriff, 1995). The modulation of ABR function during heavy and exhausting exercise, which entailed resetting MSNA control to higher blood pressures and higher MSNA levels, and increases in ABR sensitivity, are consistent with observations made during isometric exercise and postexercise muscle ischaemia (a time when the muscle metaboreflex would be activated in the absence of both central command and the muscle mechanoreflex) (Cui et al. 2001; Kamiya et al. 2001; Ichinose et al. 2004b, 2006b). Thus, it might be that the muscle metaboreflex activated at heavy and exhausting leg cycling induced these modifications of ABR function.
The muscle mechanoreflex is also thought to contribute to the cardiovascular responses seen during dynamic exercise (Mitchell, 1990; Rowell & O'Leary, 1990; Rowell et al. 1996; Nishiyasu et al. 2001) and to induce modulation of ABR function (Iellamo et al. 1997; Potts & Mitchell, 1998; Yamamoto et al. 2004). However, the effect of the muscle mechanoreflex on ABR control over MSNA in humans is still unknown. It might be initially activated at the lowest workload and become progressively more activated as workload increases. If so, the muscle mechanoreflex could be a factor in the gradual modulation of ABR function observed as exercise intensity increases (i.e. the progressive upward resetting and the increased ABR sensitivity at higher intensities).
Activation of central command reportedly induces resetting of ABR control of blood pressure and HR (Gallagher et al. 2001; McIlveen et al. 2001; Ogoh et al. 2002). In addition, we previously observed that exclusion of central command (and muscle mechanoreflex) during postexercise muscle ischaemia results in a leftward and downward shift in the DAP–MSNA relationships and a reduction in ABR sensitivity from that observed during peak isometric exercise (Ichinose et al. 2006b). According to Victor et al. (1989), central command has no effect on MSNA during mild to moderate isometric exercise, but MSNA is increased when central command is strongly activated. Thus, the strong activation of central command that would occur during heavy and exhausting exercise also could account for the upward resetting of the DAP–MSNA relations and the increase in the sensitivity of ABR control over total MSNA.
We found that MSNA remained high during the 10 min recovery period after exhaustion (burst frequency and total MSNA at Rec1 were 228 ± 29% and 435 ± 71% of the resting condition, respectively (n = 5)). What's more, the sensitivity of ABR control over total MSNA remained above the resting level. Cessation of exercise would remove central command, the muscle mechanoreflex and the metaboreflex, so that these three factors could not account for the increased MSNA and the modification of ABR function seen during recovery. At the same time, however, central blood volume would decline due to reduced venous return and blood pooling in lower body after termination of leg cycling. In addition, an increase in blood flow to the skin and a reduction in plasma volume due to sweating caused by exercise-induced hyperthermia (Rowell, 1986), and movement of fluid from the intravascular to the interstitial space (Mack et al. 1998), also could contribute to a reduction in central blood volume, which would increase MSNA by unloading of the cardiopulmonary baroreceptors (Sundlof & Wallin, 1978a; Ichinose et al. 2004a, 2006a). Consistent with that idea, we previously found that unloading the cardiopulmonary baroreceptors induces an upward shift of the DAP–MSNA relationships (Ichinose et al. 2004a, 2006a).
Another possibility is that an increase in body temperature is involved in the increase in MSNA and modulation of ABR function. Our incremental dynamic exercise must have caused substantial exercise-induced hyperthermia. Taking into account the results of previous studies (Saltin & Hermansen, 1966; Schemidt & Brück, 1981; Kenny & Niedre, 2002), we suggest core body temperature might be increased by as much as 2°C by exhaustion, and it would have remained elevated through the 10 min recovery period (Kenny & Niedre, 2002; Hayashi et al. 2004). Previous studies have shown that MSNA increases with increasing core body temperature during passive heating (Niimi et al. 1997; Cui et al. 2002; Keller et al. 2006). In addition, Saito et al. (1997) reported that MSNA gradually increases with increasing tympanic temperature during prolonged mild leg cycling, which suggests that exercise-induced hyperthermia increases MSNA. It also has been shown that increasing core body temperature by passive heating induces an upward shift in the DAP–MSNA relationship (Cui et al. 2002; Kamiya et al. 2003) as well as an increase in the sensitivity of ABR control over MSNA (Kamiya et al. 2003; Keller et al. 2006). It thus seems likely that exercise-induced hyperthermia contributed to the increase in MSNA and modulation of ABR control over MSNA seen during the exercise protocol and recovery period. That said, further studies are clearly needed to better understand the individual and interactive effects of central command and each peripheral neural input on the ABR control over MSNA during dynamic exercise in humans.
Our results obtained during the recovery periods are very different from those obtained by Halliwill et al. (1996), which is the only other data set containing MSNA measurements made after dynamic exercise that we are aware of. Those investigators reported that MSNA was ∼30% lower and the DAP–MSNA relationship was shifted downward 60 min after a 60 min bout of cycling at 60%V̇O2,max, as compared to pre-exercise resting condition. The difference between our findings and theirs could be attributable to the different intensity and duration of exercise, body posture during the recovery measurement (i.e. seated versus supine), and/or the postexercise time period when the MSNA was recorded.
We found several differences in the ABR control over burst strength and burst incidence during the incremental leg cycling. The close relationship between burst incidence and DAP was maintained from the resting period across all exercise intensities and the recovery periods. By contrast, a relatively weak relationship between burst strength and DAP was seen in all of those settings, which suggests that whereas ABR dominantly control burst incidence, input from the arterial baroreceptors is not strong enough to exert the same degree of control over burst strength. Moreover, incremental dynamic exercise modulated ABR control of burst incidence and burst strength differently. Given that ABR control over total MSNA is dependent on its control over both burst incidence and strength (Ichinose et al. 2004a,b, 2006a,b; Keller et al. 2006), the upward shift of the DAP–total MSNA relationship seen during moderate to heavy exercise would reflect the upward shift of the DAP–burst strength relationship, since the DAP–burst incidence relationship did not shift upward from the resting level. In addition, the upward shift of the burst strength line (i.e. the increased burst strength) seen during heavy and exhausting exercise could cause the total MSNA line to have a steeper slope, even in the face of a reduced slope in the burst incidence line, because the total MSNA line can be expressed as the product of the burst incidence and burst strength lines. We also observed a clear tendency toward increases in the sensitivity of burst strength control during heavy and exhausting exercise, though it was not statistically significant (during exhausting exercise, the slope of the burst strength line increased in 3 out of 4 subjects, as compared to the resting condition). Therefore the increased sensitivity of burst strength control could also account for the increased sensitivity of total MSNA control in some subjects. In sum, our results support the notion that the occurrence and strength of sympathetic bursts are differentially regulated (Malpas et al. 1996; Kienbaum et al. 2001; Ichinose et al. 2004a,b, 2006a,b). But although evidence of differential control of the occurrence and strength of sympathetic bursts has been reported (Hjemdahl et al. 1989; Malpas et al. 1996; Sverrisdottir et al. 2000; Kienbaum et al. 2001; Ichinose et al. 2004a,b, 2006a,b; Keller et al. 2006; Ogoh et al. 2007), the mechanism remains unknown (McAllen & Malpas, 1997).
Limitations
To evaluate ABR control over MSNA, we examined spontaneous fluctuations in blood pressure and MSNA in subjects at rest and during incremental exercising. There are several limitations attached to this approach. Although a linear relationship between the spontaneous fluctuations in MSNA and DAP has been demonstrated previously (Sundlof & Wallin, 1978a,b; Kienbaum et al. 2001; Kamiya et al. 2001; Ichinose et al. 2004a,b, 2006a,b), the spontaneous blood pressure fluctuations are not particularly large. This means that the ABR stimulus–response range that can be examined with this method is limited (within 20 mmHg). Consequently, we cannot discount the possibility that an increase or reduction in ABR sensitivity observed during incremental dynamic exercise resulted from a shift to a low or high gain portion of the full ABR stimulus–response curve. But although it is a narrower range than is obtained using other methods, such as the neck-chamber technique (Fadel et al. 2001; Ogoh et al. 2002; Ichinose et al. 2002) or invasive pharmacological manipulation (Cui et al. 2001, 2002; Charkoudian et al. 2004), a 20 mmHg change in blood pressure is within the physiological range and should be a good reflection of the ABR control over MSNA under physiological conditions. Furthermore, to investigate the reflex effect elicited when two or more inputs are summed (e.g. ABR and exercise pressor reflexes in this study), it is important to use inputs that are small enough not to cause saturation of the output due to the inherent limitation on the effector responses of the system (Sagawa, 1983). On that basis, our experimental results can be taken as indicative of physiological modulation of the ABR control over MSNA during dynamic exercise.
We performed the linear regression analysis with and without weighting of the data with respect to the numbers of cardiac cycles in the DAP bins. From a statistical perspective, the weighting method has merit in that weighting the data removes bias introduced because some DAP bins contained very few cardiac cycles (Kienbaum et al. 2001). On the other hand, from a physiological viewpoint, the number of cardiac cycles in each DAP bin should not be included in the calculation of ABR sensitivity because the baroreflex stimulus–response relationship is independent of the number of cardiac cycles (i.e. the baroreflex does not function to detect the number of cardiac cycles). We therefore think that both methods have advantages and limitations. In any case, we reached the same conclusions using either method.
Respiration is an important contributor to oscillations in blood pressure. Exercise induced increases in the respiration rate could cause faster oscillations in blood pressure and influence the observed changes in ABR responsiveness. The impact of changes in the respiration rate and the rate at which blood pressure was changed on ABR sensitivity assessed by our method is uncertain, and further studies will be needed to address these issues.
The position of the needle used to record nerve activity affects the MSNA measurements, especially the burst strength analysis. To confirm the quality of MSNA measurements, we carefully inspected the signal and the sound of the nerve activity during experiments and during the offline data analysis. For the data analysis, we eliminated any data that showed appreciable shifts in the baseline during cycling. As a result, we were able to obtain only five data sets that were sufficiently clear for analysis all the way up to the exhausting exercise. Nonetheless, we still cannot completely rule out the possibility that subtle changes in the needle position occurred during exercise and influenced the MSNA measurements, as we do not have a method to directly verify the needle position. Still, the curvilinear increase in MSNA with increasing exercise intensity observed in the present study is very consistent with the pattern of the rise in plasma noradrenaline levels elicited by increases in dynamic exercise intensity (Lehmann et al. 1985; Mazzeo & Marshall, 1989). In addition, the MSNA responses seen during incremental leg cycling in the present study are also consistent with earlier reports (Saito et al. 1993, 1999). We therefore believe that our MSNA measurements are qualitatively reliable.
During the 3 min data segments for heavy and exhausting exercise, we found slight gradual increases in HR and MSNA, though blood pressure remained constant. During exhausting exercise, the HR and total MSNA at the 3rd min of the segment were, respectively, < 5% and < 8%, higher than during the 1st min. Because the increase in MSNA was small, and DAP remained constant during these data segments, it is unlikely that using the non-steady-state data segments had a significant impact on the regression analysis and our conclusions.
We used absolute intensities for the incremental exercise protocol. It would have been more appropriate to use relative intensities to assess the responses to the different exercise levels; however, the relative intensity of each workload calculated as a percentage of the exhausting exercise workload in the five subjects who accomplished the entire exercise protocol was clearly differentiated with no overlaps between workloads (very mild, 5.6 ± 0.6%, range 4.3–9.0%; mild, 40.7 ± 1.4%, range 36.4–47.4%; moderate, 65.2 ± 0.6%, range 63.6–68.4%; heavy, 85.3 ± 1.0%, range 81.8–89.4%; exhausting, 100%). Therefore, even if we had used relative intensities, the results of the present study would not be changed.
In conclusion, our results show that in human subjects, ABR control over MSNA is not uniform throughout a leg cycling exercise during which intensity is incrementally increased from very mild to exhausting. We suggest that this non-uniformity of ABR function is one of the mechanisms by which sympathetic and cardiovascular responses are matched to the exercise intensity.
Acknowledgments
We would like sincerely to thank the volunteer subjects. This study was supported by grants from Center of Excellence (COE) projects, and the Ministry of Education, Science, and Culture of Japan. M. Ichinose is the recipient of a research fellowship for young scientists from Japan Society of the Promotion of Science.
References
- Bevegård S, Freyschuss U, Strandell T. Circulatory adaptation to arm and leg exercise in supine and sitting position. J Appl Physiol. 1966;21:37–46. doi: 10.1152/jappl.1966.21.1.37. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol. 1994;77:1403–1410. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1658–H1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R252–R258. doi: 10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Strømstand M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol. 2001;533:861–870. doi: 10.1111/j.1469-7793.2001.t01-1-00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;495:279–288. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Honda Y, Ogawa T, Wada H, Kondo N, Nishiyasu T. Effects of brief leg cooling after moderate exercise on cardiorespiratory responses to subsequent exercise in the heat. Eur J Appl Physiol. 2004;92:414–420. doi: 10.1007/s00421-004-1145-y. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol Endocrinol Metab. 1989;257:E654–E664. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol. 2006a;576:947–958. doi: 10.1113/jphysiol.2006.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Kondo N, Nishiyasu T. Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2006b;290:H1419–H1426. doi: 10.1152/ajpheart.00847.2005. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of control of muscle sympathetic nerve activity during orthostatic stress in humans. Am J Physiol Heart Circ Physiol. 2004a;287:H2147–H2153. doi: 10.1152/ajpheart.00215.2004. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol. 2002;544:939–948. doi: 10.1113/jphysiol.2002.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol. 2004b;286:H701–H707. doi: 10.1152/ajpheart.00618.2003. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol Heart Circ Physiol. 1997;272:H1157–H1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Fu Q, Niimi Y, Iwase S, Mano T, Suzumura A. Static handgrip exercise modifies arterial baroreflex control of vascular sympathetic outflow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1134–R1139. doi: 10.1152/ajpregu.2001.281.4.R1134. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Hayano J, Sunagawa K. Heat stress modifies human baroreflex function independently of heat-induced hypovolemia. Jpn J Physiol. 2003;53:215–222. doi: 10.2170/jjphysiol.53.215. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny GP, Niedre PC. The effect of exercise intensity on the post-exercise esophagel temperature response. Eur J Appl Physiol. 2002;86:342–346. doi: 10.1007/s00421-001-0538-4. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Schmid P, Keul J. Plasma catecholamine and blood lactate cumulation during incremental exhaustive exercise. Int J Sports Med. 1985;6:78–81. doi: 10.1055/s-2008-1025817. [DOI] [PubMed] [Google Scholar]
- Mack GW, Yang R, Hargens AR, Nagashima K, Haskell A. Influence of hydrostatic pressure gradients on regulation of plasma volume after exercise. J Appl Physiol. 1998;85:667–675. doi: 10.1152/jappl.1998.85.2.667. [DOI] [PubMed] [Google Scholar]
- Malpas SC, Bendle RD, Head GA, Ricketts JH. Frequency and amplitude of sympathetic discharges by baroreflexes during hypoxia in conscious rabbits. Am J Physiol Heart Circ Physiol. 1996;271:H2563–H2574. doi: 10.1152/ajpheart.1996.271.6.H2563. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Marshall P. Influence of plasma catecholamines on the lactate threshold during graded exercise. J Appl Physiol. 1989;67:1319–1322. doi: 10.1152/jappl.1989.67.4.1319. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Malpas SC. Sympathetic burst activity: characteristics and significance. Clin Exp Pharmacol Physiol. 1997;24:791–799. doi: 10.1111/j.1440-1681.1997.tb02693.x. [DOI] [PubMed] [Google Scholar]
- McIlveen SA, Shawn GH, Kaufman MP. Both central command and exercise pressor reflex rest carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1451–H1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- Miki K, Yoshimoto M, Tanimizu M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J Physiol. 2003;548:313–322. doi: 10.1113/jphysiol.2002.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Mitchell JH, Schmidt RF. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow, part 2. Bethesda, MD, USA: American Physiological Society; 1983. pp. 623–658. [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Sone R, Tan N, Maekawa T, Kondo N. Effects of rhythmic muscle compression on arterial blood pressure at rest and during dynamic exercise in humans. Acta Physiol Scand. 2001;173:287–295. doi: 10.1046/j.1365-201X.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle? Am J Physiol Heart Circ Physiol. 1995;268:H980–H986. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007;293:H2202–H2209. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, Yurvati AO, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543:349–364. doi: 10.1113/jphysiol.2002.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Mitchell JH. Rapid resetting of carotid baroreflex by afferent input from skeletal muscle receptors. Am J Physiol Heart Circ Physiol. 1998;275:H2000–H2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol. 1993;264:H1–H7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation. New York: Oxford University Press; 1986. [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflex and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS, Kellog DL. Integration of cardiovascular control systems in dynamic exercise. In: Rowell LB, editor. Handbook of Physiology, section 12, Exercise: Regulation & Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 1025–1063. [Google Scholar]
- Sagawa K. Baroreflex control of systemic arterial pressure and vascular beds. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III Peripheral Circulation and Organ Blood Flow, part 2. Bethesda, MD, USA: American Physiological Society; 1983. pp. 453–496. [Google Scholar]
- Saito M, Kanao Y, Tanaka H, Sakai T. Muscle sympathetic nerve responses during progressive cycling exercise. Adv Exerc Sports Physiol. 1999;5:19–25. [Google Scholar]
- Saito M, Mano T. Exercise mode affects muscle sympathetic nerve responsiveness. Jpn J Physiol. 1991;41:143–151. doi: 10.2170/jjphysiol.41.143. [DOI] [PubMed] [Google Scholar]
- Saito M, Sone R, Ikeda M, Mano T. Sympathetic outflow to the skeletal muscle in humans increases during prolonged light exercise. J Appl Physiol. 1997;82:1237–1243. doi: 10.1152/jappl.1997.82.4.1237. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21:1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Schemidt V, Brück K. Effect of a precooling maneuver on body temperature and exercise performance. J Appl Physiol. 1981;50:772–778. doi: 10.1152/jappl.1981.50.4.772. [DOI] [PubMed] [Google Scholar]
- Stenberg J, Astrand PO, Ekblom B, Royce J, Saltin B. Hemodynamic response to work with different muscle groups, sitting and supine. J Appl Physiol. 1967;22:61–70. doi: 10.1152/jappl.1967.22.1.61. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol. 1978a;278:525–532. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978b;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverrisdottir YB, Rundqvist B, Johannsson G, Elam M. Sympathetic neural burst amplitude distribution: a more specific indicator of sympatho-excitation in human heart failure. Circulation. 2000;102:2076–2081. doi: 10.1161/01.cir.102.17.2076. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol. 1989;257:H2017–H2024. doi: 10.1152/ajpheart.1989.257.6.H2017. [DOI] [PubMed] [Google Scholar]
- Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol. 1983;245:H481–H486. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kawada T, Kamiya A, Takaki H, Miyamoto T, Sugimachi M, Sunagawa K. Muscle mechanoreflex induces the pressor response by resetting the arterial baroreflex neural arc. Am J Physiol Heart Circ Physiol. 2004;286:H1382–H1388. doi: 10.1152/ajpheart.00801.2003. [DOI] [PubMed] [Google Scholar]