Abstract
This study was done to test the hypothesis that simulated vaginal birth by vaginal distension (VD) causes more severe urinary incontinence and slower recovery in diabetic rats. After measuring baseline leak point pressure (LPP) in 16 diabetes mellitus (DM) and 16 age- and weight-matched control (Ct) female Sprague-Dawley rats, these animals underwent either VD or sham VD (sham). Four and ten days after the procedures, LPP and conscious cystometry were assessed. Tissues were then harvested and examined by light microscopy. LPP at baseline was equal among all four groups. Four days after VD, LPP in both VD groups dropped to significantly lower levels than in sham rats (P < 0.001). Moreover, LPP in the DM+VD group was significantly lower than in the Ct+VD group. At 10 days, LPP in the Ct+VD group had recovered to its baseline value, whereas the LPP in the DM+VD group remained significantly reduced. DM rats had larger bladder capacity and longer voiding intervals than Ct rats. Histological findings included more severe damage to the external sphincter striated musculature of the urethra in DM+VD group compared with Ct+VD. In conclusion, these findings suggest that DM causes increased severity and delayed functional recovery from the effects of simulated childbirth.
Keywords: vaginal distension, leak point pressure, diabetic complications, simulated childbirth animal model
Diabetes Mellitus (Dm) Causes debilitating and devastating complications, including urinary incontinence (4). Among patients with either type 1 or 2 DM, women have a higher prevalence of lower urinary tract (LUT) complications, contributing to the high prevalence of urinary incontinence (30-60%) among U.S. women (28). Brown et al. (4) reported a significantly higher prevalence of urinary incontinence (UI) in diabetic women compared with nondiabetic women in a sample of 2,763 postmenopausal women, comparable to the results of other investigators (29, 32). However, the mechanistic relationship of increased risk for UI among diabetic women remains poorly understood.
Trauma to the pelvic floor during vaginal delivery of children is a recognized risk factor for urinary incontinence in older women (41). It is plausible that diabetic women, because of preexisting alterations in their lower urinary tract/vaginal tissues, such as autonomic neuropathy or mypoathy, may sustain greater injury and do not recover as well from the birth trauma as nondiabetic women. Therefore, we hypothesize that pelvic floor trauma during vaginal childbirth induces greater injury and slower recovery in diabetics than in normal women.
For mechanistic studies of the association between vaginal delivery and UI, several investigators have used simulated childbirth models in rats by vaginal distension (VD). VD causes damage to the urinary continence mechanism, decreased urethral resistance, and symptoms that mimic clinical stress urinary incontinence (11, 25, 34). The current study was designed to test the hypothesis that simulated vaginal birth by VD causes more severe urinary incontinence and slower recovery in diabetic rats.
MATERIALS AND METHODS
Diabetes was produced in 16 virgin female Sprague-Dawley rats (Harlan, 250-300 g body wt) by intraperitoneal injection of streptozotocin (50-60 mg/kg) after a 24 h fast and was confirmed by blood glucose levels greater than 300 mg/dl. After 6-8 wk of diabetes, the 16 diabetic animals and 16 age-matched control rats were randomized to receive either VD or sham distension, creating 4 groups: 1) DM with vaginal distension (DM+VD); 2) DM with sham distension (DM+Sham); 3) Control with vaginal distension (Ct+VD); and 4) Control with sham distension (Ct+Sham). All animals were housed in separate cages throughout the course of the experiments. This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Lerner Research Institute of the Cleveland Clinic Foundation.
Three days before VD or sham distension, each rat received a chronic implant of a suprapubic bladder catheter for urodynamic testing. Bladder capacity and leak point pressure were measured just prior to VD, as well as 4 and 10 days after VD or sham distension. Conscious cystometry was performed 4 and 10 days after VD or sham distension. At the completion of all studies, 10 days after distension, the animals were euthanized and the bladder, urethra, and vagina were dissected and prepared for histological analysis.
Suprapubic Tube Implantation
Suprapubic tube (SPT) insertion was performed as modified from previous work (11). In brief, the rats were anesthetized by an intraperitoneal injection of a mixture of ketamine (100 mg/kg body wt) and xylazine (15 mg/kg body wt). A purse-string suture with 6-0 silk was placed on the bladder dome and a 1-mm incision was made in the center. Polyethylene (PE)-50 tubing with a flared tip was then implanted, and the purse string was tightened around the tube. The SPT was tunneled under the skin to exit between the ears.
Bladder Capacity and Leak Point Pressure Testing
Bladder capacity and leap point pressure (LPP) were measured under anesthesia as described by Cannon and Damaser (6) 3 days after SPT implantation just prior to VD, as well as 4 and 10 days after VD (6). The rats were anesthetized as above with ketamine and xylazine, placed supine at the level of zero pressure, and the bladder was emptied manually. Subsequently, the bladder was filled with saline at room temperature (6 ml/h) through the SPT, while bladder pressure was recorded. The SPT was connected to both a syringe pump (Pump 11 Plus Advanced Single Syringe with Dual RS-23, Harvard Apparatus, Holliston, MA) and a pressure transducer (FT10, Astro-Med Inc, Providence, RI). All bladder pressures were referenced to air pressure at the level of the bladder. Pressure signals were amplified (LP122 series, Astro-Med), and digitized for computer data collection (PolyVIEW/SYS, Astro-Med, West Warwick, RI) at 10 samples/s.
Bladder capacity was measured as the filled volume at the first sign of visible leakage. The bladder was then emptied and the bladder was filled again. Capacity was measured 3 times, and the mean value was calculated for use in further analysis. LPP was determined as the peak bladder pressure inducing urethral leakage by slowly applying manual pressure to the lower abdomen at half bladder capacity, as we have done previously (6). The LPP was tested 3-5 times on each rat, and the mean was calculated for use in further data analysis. The bladder was emptied using the Crede maneuver and refilled to approximately half capacity between LPP measurements. Previous work has demonstrated that LPP is not sensitive to the volume at which it is measured (6).
Vaginal Distension
Vaginal distension was performed as modified from previous work (7, 11). The rats were anesthetized as above with ketamine and xylazine. To avoid rupturing the vagina, it was first accommodated to a larger capacity by subsequently inserting and removing increasing sizes of urethral dilators (24 Fr to 32 Fr), lubricated with surgilube (E. Fougera, New York). A modified 10-Fr Foley catheter was then lubricated and inserted into the vagina. The balloon was distended with water inside the vagina to 2 ml in rats weighing 100-200 g and 3 ml in rats weighing over 200 g, since our preliminary studies indicated that vaginal capacity was a function of rat size. After 3 h, the catheter was deflated and removed, and the animals were allowed to awaken from the anesthesia.
Animals in the sham distension groups were also anesthetized and had the vagina accommodated using the urethral dilators. A catheter was inserted into the vagina; however, it was not inflated.
Conscious Cystometry
Conscious cystometry (CMG) testing was performed as done previously (6). Four and ten days after either VD or sham procedure, the rats were placed in specially modified metabolic cages for a period of 2-5 h. During this time, the SPT was attached via a stopcock to both a pressure transducer and a flow pump. The bladder was then filled with room temperature saline (6 ml/h) via the catheter, while bladder pressure was recorded. The animals were awake and able to void and/or leak urine through the urethra during the study. Urine was collected in a beaker on a force transducer (Model PT5, Astro-Med,) placed beneath each cage. The pressure and force transducers were connected to an amplifier, chart recorder, and a computer for recording data as described above. The bladder was filled 3-5 times in each animal, and the mean of each variable was taken for use in further analysis. After cystometry, the animal was anesthetized and underwent LPP testing, as described above.
The following variables were measured from CMG data: threshold pressure, peak voiding pressure, resting pressure, compliance, and mean intercontractile interval. Peak voiding pressure was defined as the maximum pressure during micturition. Threshold pressure was defined as the recorded pressure just prior to the onset of micturition phase. Resting pressure was defined as the pressure recorded just after the end of micturition. Compliance was calculated by dividing the voided volume by the change in bladder pressure during the voiding phase (threshold pressure minus the resting pressure). The mean intercontractile interval was defined as time between the start of one voiding cycle (at the beginning resting pressure) to the end of that voiding cycle (at the end of the micturition phase).
Histology
Ten days after VD, the animals were euthanized immediately following LPP testing. The bladder, urethra, and vagina were harvested, immersion fixed in 10% neutral buffered formalin, and embedded in paraffin. Full-thickness cross sections of bladder, urethra, and vagina (5 μm) were deparaffinized, hydrated with distilled water and stained with hematoxylin & eosin; Masson’s trichrome, or Movat pentachrome. For anatomical assessment, tissues of three animals per group were analyzed and compared.
Data Analysis
Means and medians were used to evaluate the central tendency of the data, while standard deviation and interquartile ranges (25th and 75th percentiles) were used to assess variability. These summary measures were also calculated for the change in levels between time points. Two animals in the DM+VD group did not survive after placement of SPT, so only six animals in that group were available for analysis. Also, only data at baseline and 4 days postdistension were available from three of the animals in the DM+Sham group who did not survive to 10 days after distension. Nonparametric tests were performed to minimize the impact of a few extreme observations on the statistical results. To evaluate differences between groups at each time point, the nonparametric Kruskal-Wallis test was performed. If the Kruskal-Wallis test indicated a significant difference (P < 0.05), then the Wilcoxon rank sum test was performed for each pair of groups with P < 0.05, indicating a significant difference. Correction of the significance level to compensate for multiple comparisons was not performed due to the small number of animals in this study. Reducing the individual significance criterion to control the overall error level may obscure differences that could be potentially interesting to study further. All analyses were performed using SAS software (Cary, NC). Histological data were analyzed qualitatively.
RESULTS
Functional Results
LPP
At the time of the vaginal distention or sham procedure, there was no significant difference in weight between the two control groups (Ct+Sham and Ct+VD) or between the two diabetic groups (DM+Sham and DM+VD). However, the diabetic animals weighed a median of 205 g with 25 and 75% quartile values of 195 and 213 g, respectively, significantly less than the control animals (median: 257 g; 25%: 250 g; 75%: 288 g). By the criteria we set out in advance to scale distension to animal size, all animals received 3 ml distension.
Baseline LPP prior to VD was similar in all four groups: 36.8 cmH2O (Ct+VD), 35.5 cmH2O (Ct+Sham), 34.6 cmH2O (DM+VD), and 35.7 cmH2O (DM+Sham). Sham distension did not significantly alter LPP in either diabetic or control animals (Fig. 1). LPP measured 4 days after VD was significantly decreased compared with LPP 4 days after a sham distension in both diabetic and nondiabetic animals (P < 0.001). Ten days after VD, LPP in the diabetic animals remained significantly decreased compared with the 3 other groups, demonstrating that symptomatic recovery in the diabetic animals is slowed.
Fig. 1.
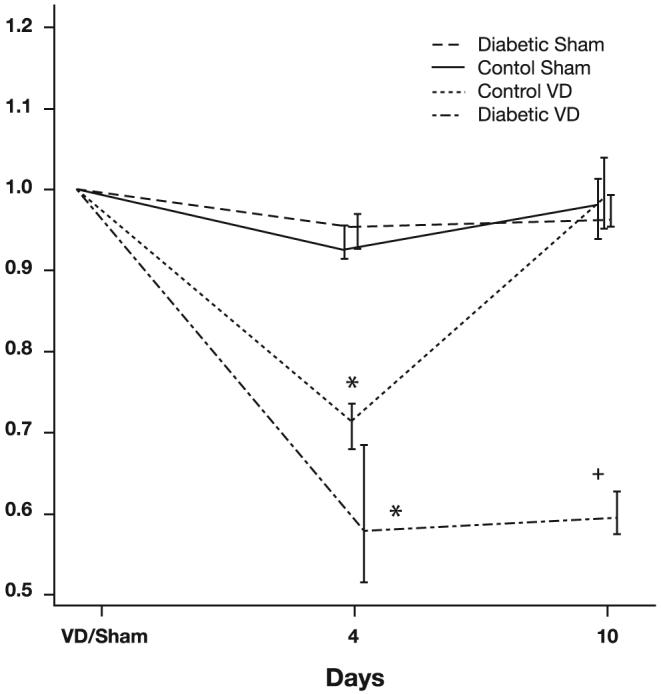
Leak point pressure (LPP) normalized to preoperative LPP values in the four study groups. Data are represented as median LPP and interquartile ranges of normalized LPP values. Each data point represents data from 5-8 animals with diabetes mellitus or controls that underwent vaginal distension (VD) or sham distension (control VD). *Significant difference compared with comparable sham animals at the same time point. +Significant difference compared with control animals at the same time point.
Conscious CMG
Four days after VD, there was a statistically significant difference in bladder capacity measured during conscious cystometry between control and diabetic sham distension groups comparable to our previous studies of diabetic cystopathy (13) (Table 1). However, the threshold pressure, peak voiding pressure, resting pressure, and compliance demonstrated no statistically significant differences between groups at any of the time points studied, possibly related to the high variability seen within treatment groups (Table 1). Both diabetic groups had larger mean intercontractile intervals than the Ct+Sham group 4 days after distension, consistent with increased capacity (Table 1). Ten days after distension, no statistically significant differences were present, although, as with capacity, the values of the DM+Sham group were higher than that of the DM+VD group. No statistically significant differences between groups between 4 and 10 days were observed.
Table 1.
Cystometry outcomes by group and time
| Median |
|||
|---|---|---|---|
| Group | Preoperative | 4 Days | 10 Days |
| Bladder Capacity, ml (anesthetized) | |||
| Ct+Sham | 0.59 (0.27, 0.88)* | 0.40 (0.30, 1.13)* | 0.90 (0.65, 1.28) |
| Ct+VD | 0.36 (0.30, 0.45)† | 0.44 (0.23, 0.84)† | 0.56 (0.33, 1.56) |
| DM+Sham | 2.30 (1.15, 3.21)*† | 2.21 (1.19, 2.82)*† | 1.32 (1.02, 1.92) |
| DM+VD | 1.44 (1.07, 1.85)*† | 1.93 (1.25, 2.07)*† | 2.05 (1.06, 5.12) |
| Bladder Capacity, ml (awake) | |||
| Ct+Sham | 0.59 (0.42, 0.65)‡ | 0.50 (0.39, 1.22) | |
| Ct+VD | 0.78 (0.36, 1.11) | 0.48 (0.38, 0.89) | |
| DM+Sham | 1.53 (0.96, 1.89)‡ | 1.55 (0.55, 3.01) | |
| DM+VD | 1.25 (0.65, 2.31) | 0.54 (0.31, 1.74) | |
| Threshold Pressure, cmH2O | |||
| Ct+Sham | 16.9 (12.5, 20.1) | 16.9 (14.2, 25.6) | |
| Ct+VD | 13.6 (11.0, 15.6) | 15.7 (12.9, 23.9) | |
| DM+Sham | 12.5 (11.5, 19.3) | 19.2 (9.7, 28.0) | |
| DM+VD | 15.6 (10.5, 22.1) | 12.9 (8.8, 15.9) | |
| Peak Voiding Pressure, cmH2O | |||
| Ct+Sham | 45.6 (46.8, 53.7) | 66.2 (45.8, 85.9) | |
| Ct+VD | 42.4 (36.7, 49.1) | 55.9 (43.0, 78.1) | |
| DM+Sham | 48.0 (46.1, 54.1) | 66.9 (42.0, 111.1) | |
| DM+VD | 51.5 (23.1, 60.3) | 53.0 (20.7, 55.3) | |
| Resting Pressure, cmH2O | |||
| Ct+Sham | 10.1 (7.6, 14.5) | 12.1 (6.4, 20.1) | |
| Ct+VD | 9.9 (7.2, 10.2) | 8.7 (6.2, 12.0) | |
| DM+Sham | 7.0 (2.9, 8.7) | 5.5 (2.7, 17.8) | |
| DM+VD | 9.0 (6.0, 18.3) | 9.7 (6.0, 10.8) | |
| Compliance, ml/cmH2O | |||
| Ct+Sham | 0.13 (0.08, 0.19) | 0.08 (0.05, 0.20) | |
| Ct+VD | 0.22 (0.06, 0.42) | 0.08 (0.07, 0.11) | |
| DM+Sham | 0.19 (0.12, 0.27) | 0.28 (0.11, 0.59) | |
| DM+VD | 0.61 (0.15, 1.36) | 0.18 (0.11, 0.62) | |
| Mean Intercontractile Interval, min | |||
| Ct+Sham | 3.87 (2.74, 4.50)§ | 2.97 (2.33, 7.30) | |
| Ct+VD | 4.65 (2.14, 6.94) | 2.85 (2.27, 5.34) | |
| DM+Sham | 9.15 (5.75, 11.31)§ | 9.27 (3.28, 18.00) | |
| DM+VD | 7.48 (4.63, 13.88)§ | 3.25 (1.83, 10.47) | |
Values are medians with percentages in parentheses (25%, 75%).
The control with sham distension (Ct+Sham) group had significantly lower bladder capacity than the diabetes mellitus with sham distention (DM+Sham) and DM with vaginal distension (DM VD) groups preoperatively and at 4 days postoperatively.
The control with vaginal distension (Ct+VD) group had significantly lower bladder capacity than the DM+Sham and DM+VD groups preoperatively and at 4 days postoperatively.
The Ct+Sham group had significantly lower bladder capacity than the DM+Sham group 4 days postoperatively.
The Ct+Sham group had significantly lower mean intercontractile intervals than both the DM+Sham and DM+VD groups at 4 days postoperatively.
Anatomical results
Striated muscle of the urethra of control animals is compact and nearly complete circumferentially with little collagen infiltration (Figs. 2 and 3). VD resulted in mild focal disruption of striated muscle fibers in control animals with structural maintenance of both striated and smooth muscle bundles (Fig. 2). The midurethra specimens from diabetic animals that had undergone VD, on the other hand, suffered extensive damage to tenuous-appearing striated muscles fibers. Moreover, there was obvious thinning and atrophy of the urethro-vaginal septum in diabetic animals. Movat stained specimen demonstrated focal increased deposition of yellow collagen fibers around the striated muscle of the urethra of diabetic animals compared with those of control animals (Fig. 3).
Fig. 2.
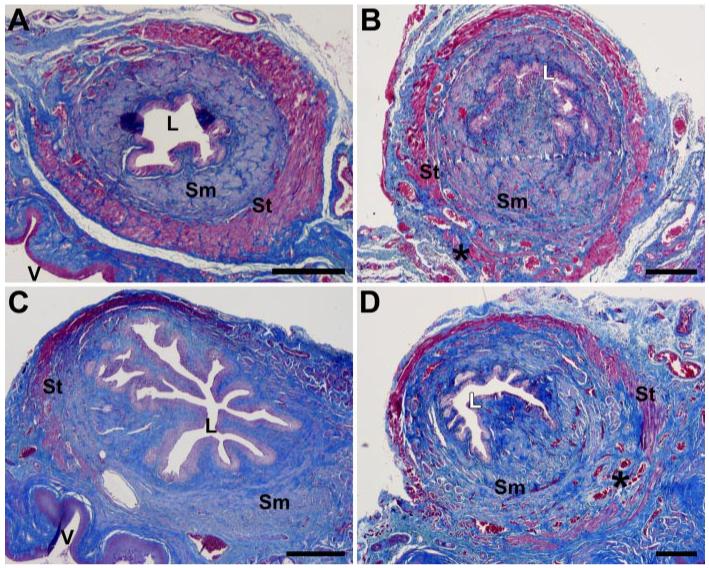
Light microscopy of urethra and anterior vagina in control rats 10 days after a sham distension (A) or a vaginal distension (B) and in diabetic rats 10 days after a sham distension (C) or a vaginal distension (D). Masson’s trichrome; *, muscle disruption; L, urethral lumen; Sm, smooth muscle; St, skeletal/striated muscle; V, vaginal lumen. Scale bar = 250 μm.
Fig. 3.
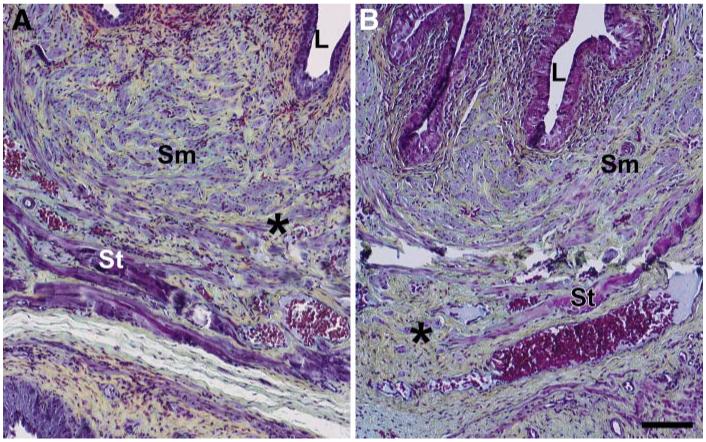
Light microscopy of urethra in control rats 10 days after a vaginal distension (A) and in diabetic rats 10 days after a vaginal distension (B). Movat pentachrome; *, collagen deposition in yellow in A and increased deposition of collagen around the smooth muscle fibers in B; L, urethral lumen; Sm, smooth muscle; St, skeletal/striated muscle. Scale bar = 100 μm.
The bladders of diabetic animals qualitatively and by gross examination demonstrated the increased capacities of diabetic animals (Fig. 4). Consistent with our previous findings in the diabetic animals (27), there appeared to be smooth muscle hypertrophy in the diabetic bladder specimens regardless of vaginal distension status. Trabeculation was noted in bladders from some diabetic animals; however, this result was not consistent across all animals.
Fig. 4.
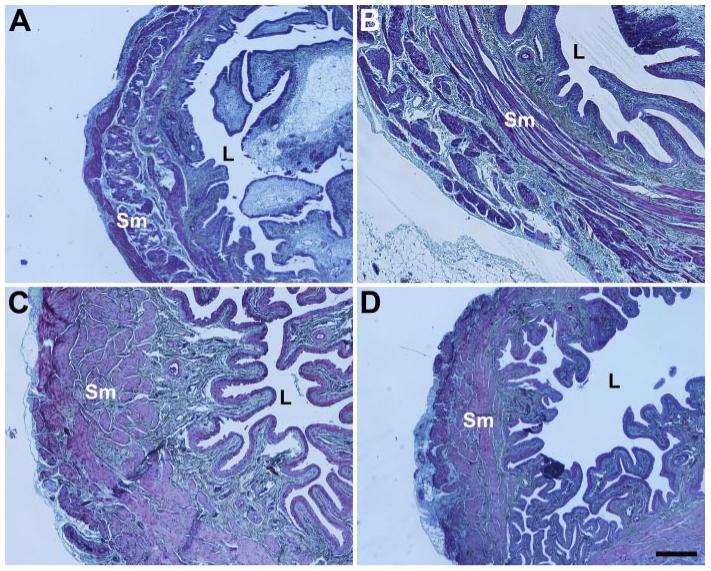
Light microscopy of bladder in control rats 10 days after a sham distension (A) or a vaginal distension (B) and in diabetic rats 10 days after a sham distension (C) or a vaginal distension (D). Movat pentachrome; L, bladder lumen; Sm, smooth muscle. Scale bar = 250 μm.
DISCUSSION
Up to one-half of U.S. women may experience some level of UI for some period of their lives (38, 44). Women with either type 1 or 2 DM have a higher prevalence of UI than nondiabetic women, as shown in several studies (4, 20, 32, 35). The Nurse’s Health Study, a prospective cohort of 121,700 women followed for almost 30 years, revealed that for severe incontinence (weekly urine leakage enough to wet the underwear) there was an almost twofold elevation in risk for women with DM (20).
The risk conferred by DM therefore appears to be additional to other recognized risk factors for UI such as age, body mass index, cigarette smoking, physical activity, parity, functional impairment, and cardiovascular disease (5). Among diabetic subjects, the risk for urge incontinence was increased by 40 to 80% in a multivariate analysis that controlled for stroke and other chronic medical conditions (3, 15). Therefore, recent and continuing rises in age-specific incidence and prevalence of DM, in the context of a demographically aging U.S. population, are likely to fuel a dramatic rise in UI incidence among DM women (4, 35, 44).
Current theories on the pathophysiology of lower urinary tract complications of DM include dysfunction of neuropathic and myopathic components of the pelvic floor (9, 10, 14, 23, 26, 33). Vaginal delivery can injure the same nerve, muscle, and connective tissues of the pelvic floor, which are responsible for maintaining continence (16, 30, 31, 36, 40).
Studies reporting that UI increases considerably during pregnancy and after delivery (1, 21, 39, 42, 43) also indicate that a large portion of these women recover from symptoms of UI within 12 mo after delivery. In a report by Hvidman et al. (24), prevalence of UI immediately after childbirth and 6 mo afterward was 23.4% and 2.7%, respectively. Mode of delivery also plays a role in the resolution of UI after delivery (18, 19). Prevalence of UI 6 mo after delivery is 10% after cesarean section, 22% after spontaneous vaginal delivery and 33% after forceps delivery (18, 19).
To study the mechanistic role of vaginal delivery on continence mechanisms, over the last few years, several investigators have developed and tested animal models in the female rat, including a VD model (11, 25, 34). These investigators have also developed a method in rats of mimicking a LPP test, the standard clinical method of diagnosing stress UI in women (6). Four days after either 1 h VD, both LPP and the increase in abdominal pressure required for leakage to occur (Pabd) were significantly decreased compared with controls (11). Those studies have shown that the decrease in LPP recovers to its baseline 10 days after injury in normal female rat.
On the basis of the above studies, we decided to use VD as a birth trauma model and also designed our experiments to measure the LPP 4 and 10 days after VD. This design would have allowed us to compare the severity (at 4 days) and recoverability (at 10 days) of the LPP between the diabetic and nondiabetic animals. We hypothesized that diabetes causes increased severity of and slowed recovery from UI after childbirth injuries sustained from VD. The damaging effect of simulated childbirth on the continence mechanism was demonstrated by the drop in LPP 4 days after VD compared with the sham groups. Ten days after VD, LPP returned to baseline in the control group but not in the diabetic group.
Assessment of cystometric changes was a side interest of this study, as we (10, 12, 13, 37) and others (2, 15, 16, 18, 22, 32, 33, 48) have published the details of the diabetic bladder dysfunction in experimental models. The results of the current study is in line with our previous findings in the small animal models of DM, which showed larger bladder capacity among the diabetic animals (12, 13). Although the observed effects of birth trauma 10 days after VD or Sham VD on the bladder function (capacity, compliance, threshold pressure, and peak voiding pressure) in diabetic animals did not reach statistical significance (Table 1, the far right column); this may warrant future exploration of role of VD on altered bladder function as it contributes to the postpartum urinary incontinence.
The results of our study should be interpreted by awareness of its limitations. First, a rat may not be the ideal model for modeling of SUI in humans. Rats are quadrupeds; they have tails with associated musculature, and their bladders are abdominal rather than pelvic organs. However, we selected the rat because it is a small animal and because incontinence and decreased urethral resistance in this model has already been shown to be easily and reproducibly induced (7, 11). In addition, vaginal distension has been shown to be an accepted model of simulated birth trauma (7, 11, 25, 34). Even though LPP measured by abdominal crede may not fully represent the mechanism of the SUI in humans, it provides an indicator of urethral resistance and bladder outlet function (6, 7, 11, 22).
The importance of this study is that it provides the first line of experimental evidence in support of a mechanistic relationship between the increased risk, severity, and delay in recovery of UI in females exposed to vaginal distension.
Several layers of evidence suggest that DM-related accumulation of reactive oxygen species (ROS) and advanced glycocylation end products (AGEs) and tissue ischemia can interactively or independently contribute to the neuropathic and myopathic causes of LUT dysfunctions (2, 8, 17). Investigation of the role of such mechanisms in our model requires further investigation.
Conclusions
The findings of the current study provide the first line of evidence toward a possible link between vaginal delivery, urinary incontinence, and diabetes in females. These findings support a hypothesis that diabetes is associated with more severe incontinence, and a delayed recovery from the injuries to the continence mechanisms in female rats undergoing vaginal distension as a surrogate of childbirth injuries. Future studies are needed to test our mechanistic hypothesis that preexisting neuropathy and other effects of prolonged hyperglycemia (such as ROS or AGE) limits the ability of lower urinary tract muscles and associated nerves to recover from the injuries sustained during vaginal delivery.
Acknowledgments
GRANTS
This study was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants KO8 DK02631, U01-DK61018-02S1, R21 DK070905-01, and R21 DK071143-01, as well as a Young Investigator Award from the National Kidney Foundation, the Diabetic Association of Greater Cleveland, and the Rehabilitation Research and Development Service of the Department of Veterans Affairs.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstet Gynecol. 2006;108:873–878. doi: 10.1097/01.AOG.0000233172.96153.ad. [DOI] [PubMed] [Google Scholar]
- 2.Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology. 2004;64:1062–1067. doi: 10.1016/j.urology.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Brown JS, Seeley DG, Fong J, Black DM, Ensrud KE, Grady D, Study of Osteoporotic Fractures Research Group Urinary incontinence inolder women: who is at risk? Obstet Gynecol. 1996;87:715–721. doi: 10.1016/0029-7844(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 4.Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001-2002. Diabetes Care. 2006;29:1307–1312. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–746. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403–407. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 8.Changolkar AK, Hypolite JA, DiSanto M, Oates PJ, Wein AJ, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol. 2005;173:309–313. doi: 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 9.Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S41–S55. doi: 10.1038/sj.bjp.0706627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christ GJ, Hsieh Y, Zhao W, Schenk G, Venkateswarlu K, Wang HZ, Tar MT, Melman A. Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int. 2006;97:1076–1082. doi: 10.1111/j.1464-410X.2006.06058.x. [DOI] [PubMed] [Google Scholar]
- 11.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 12.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1728–R1735. doi: 10.1152/ajpregu.00654.2005. [DOI] [PubMed] [Google Scholar]
- 13.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006;176:380–386. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- 14.Daneshgari F, Moore C. Diabetic uropathy. Semin Nephrol. 2006;26:182–185. doi: 10.1016/j.semnephrol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Diokno AC, Brock BM, Herzog AR, Bromberg J. Medical correlates of urinary incontinence in the elderly. Urology. 1990;36:129–138. doi: 10.1016/0090-4295(90)80211-5. [DOI] [PubMed] [Google Scholar]
- 16.Dolan LM, Hosker GL, Mallett VT, Allen RE, Smith AR. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG. 2003;110:1107–1114. [PubMed] [Google Scholar]
- 17.Eika B, Levin RM, Longhurst PA. Collagen and bladder function in streptozotocin-diabetic rats: effects of insulin and aminoguanidine. J Urol. 1992;148:167–172. doi: 10.1016/s0022-5347(17)36546-1. [DOI] [PubMed] [Google Scholar]
- 18.Farrell SA, Allen VM, Baskett TF. Parturition and urinary incontinence in primiparas. Obstet Gynecol. 2001;97:350–356. doi: 10.1016/s0029-7844(00)01164-9. [DOI] [PubMed] [Google Scholar]
- 19.Foldspang A, Hvidman L, Mommsen S, Nielsen JB. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923–927. doi: 10.1111/j.0001-6349.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 20.Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428–434. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 21.Groutz A, Helpman L, Gold R, Pauzner D, Lessing JB, Gordon D. First vaginal delivery at an older age: Does it carry an extra risk for the development of stress urinary incontinence? Neurourol Urodyn. 2007;83:941–945. doi: 10.1002/nau.20414. [DOI] [PubMed] [Google Scholar]
- 22.Hijaz A, Daneshgari F, Huang X, Bena J, Liu G, Saffore L, Damaser M. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol. 2005;174:771–775. doi: 10.1097/01.ju.0000164721.52278.29. [DOI] [PubMed] [Google Scholar]
- 23.Hunter KF, Moore KN. Diabetes-associated bladder dysfunction in the older adult (CE) Geriatr Nurs. 2003;24:138–145. doi: 10.1067/mgn.2003.49. [DOI] [PubMed] [Google Scholar]
- 24.Hvidman L, Foldspang A, Mommsen S, Nielsen JB. Postpartum urinary incontinence. Acta Obstet Gynecol Scand. 2003;82:556–563. doi: 10.1034/j.1600-0412.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288:F1220–F1226. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837–R843. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 28.Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496–1501. doi: 10.1067/mob.2001.114868. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama H, Jorgensen HS, Pedersen PM, Raaschou HO, Olsen TS. Prevalence and risk factors of incontinence after stroke. The Copenhagen Stroke Study. Stroke. 1997;28:58–62. doi: 10.1161/01.str.28.1.58. [DOI] [PubMed] [Google Scholar]
- 30.Rempen A, Kraus M. Measurement of head compression during labor: preliminary results. J Perinat Med. 1991;19:115–120. doi: 10.1515/jpme.1991.19.1-2.115. [DOI] [PubMed] [Google Scholar]
- 31.Retzky SS, Rogers RM., Jr. Urinary incontinence in women. Clin Symp. 1995;47:2–32. [PubMed] [Google Scholar]
- 32.Sampselle CM, Wyman JF, Thomas KK, Newman DK, Gray M, Dougherty M, Burns PA, Association of Women’s Health Obstetric and Neonatal Nurses Continence for women: evaluation of AWHONN’s third research utilization project. J Obstet Gynecol Neonatal Nurs. 2000;29:9–17. doi: 10.1111/j.1552-6909.2000.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am. 2003;30:1–12. doi: 10.1016/s0094-0143(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 34.Sievert KD, Emre BM, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 35.Smith DB. Urinary incontinence and diabetes: a review. J Wound Ostomy Continence Nurs. 2006;33:619–623. doi: 10.1097/00152192-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet. 1984;2:546–550. doi: 10.1016/s0140-6736(84)90766-9. [DOI] [PubMed] [Google Scholar]
- 37.Steers WD, Mackway AM, Ciambotti J, de Groat WC. Effects of streptozotocin-induced diabetes on bladder function in the rat. J Urol. 1990;143:1032–1036. doi: 10.1016/s0022-5347(17)40177-7. [DOI] [PubMed] [Google Scholar]
- 38.Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998;46:473–480. doi: 10.1111/j.1532-5415.1998.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 39.Thomason AD, Miller JM, DeLancey JO. Urinary incontinence symptoms during and after pregnancy in continent and incontinent primiparas. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:147–151. doi: 10.1007/s00192-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 40.Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest. 1996;41:132–134. doi: 10.1159/000292059. [DOI] [PubMed] [Google Scholar]
- 41.Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest. 1996;41:132–134. doi: 10.1159/000292059. [DOI] [PubMed] [Google Scholar]
- 42.Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol. 2006;108:248–254. doi: 10.1097/01.AOG.0000226860.01127.0e. [DOI] [PubMed] [Google Scholar]
- 43.Wesnes SL, Rortveit G, Bo K, Hunskaar S. Urinary incontinence during pregnancy. Obstet Gynecol. 2007;109:922–928. doi: 10.1097/01.AOG.0000257120.23260.00. [DOI] [PubMed] [Google Scholar]
- 44.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]


