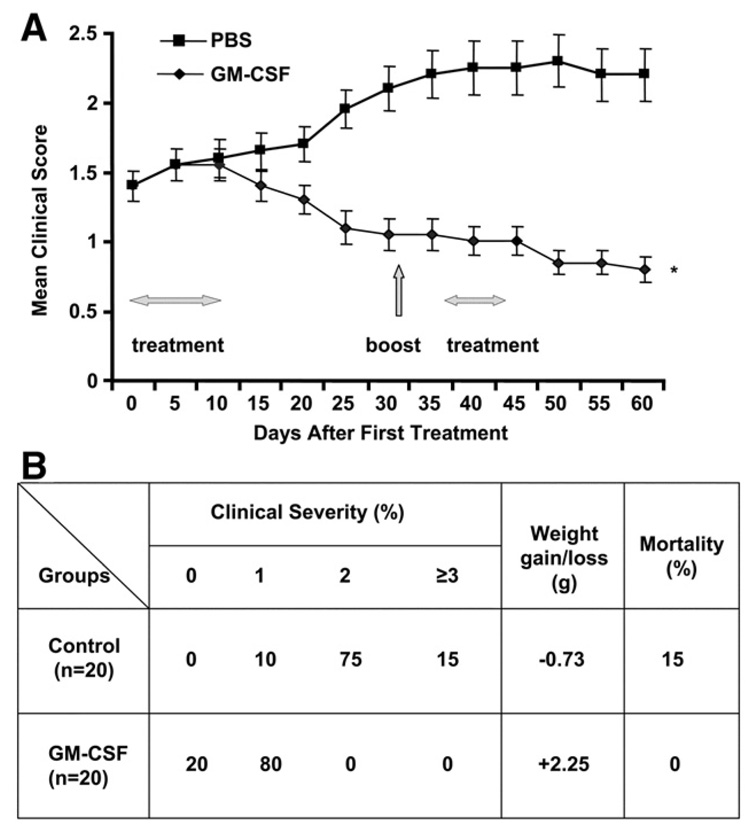
Figure 1.
Frequency and severity of EAMG in GM-CSF-treated, compared to PBS-treated, tAChR-immunized mice. Mice were evaluated as described in Materials and methods on an every other day basis beginning at the time of initiation of treatment (Day 0). A) Average clinical score during days 0–60 of the observation period is shown for the two groups (n=20/group). Day 0 corresponds to initiation of treatment (GM-CSF vs. PBS). All mice had received a priming immunization and 3 booster immunizations with tAChR. An additional tAChR booster immunization was given on day 32, after treatment initiation as shown in panel 1. Greater than 90% of animals had clinically demonstrable disease, and the GM-CSF and PBS groups had equal distribution of disease severity at day 0. B) Proportion of animals with each clinical disease severity grade, weight gain/loss, and mortality in each experimental group at the endpoint (Day 60) is shown. Disease severity, percentage weight loss, and mortality were significantly lower in GM-CSF-treated mice compared to the PBS-treated tAChR-immunized controls (*p<0.01). The above experiment was initially performed utilizing smaller numbers in each group (n=8) with similar results.