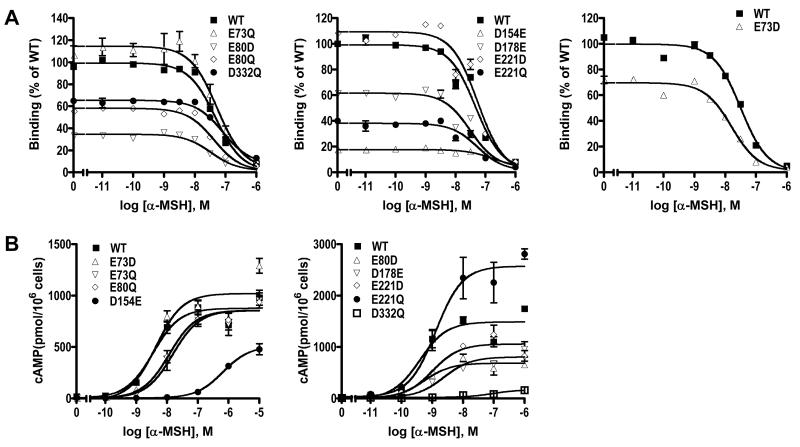
Figure 5.
Ligand binding and signaling properties of the WT and mutant hMC3Rs with α-MSH as the ligand. HEK293T cells were transiently transfected with the indicated hMC3R constructs and binding and signaling properties of the hMC3Rs were measured as described in Materials and Methods. In A, different concentrations of unlabeled α-MSH were used to displace the binding of 125I-NDP-MSH to hMC3Rs on intact cells. Results shown are expressed as % of WT binding ± range from duplicate determinations within one experiment. In B, HEK293T cells transiently transfected with the indicated hMC3R constructs were stimulated with different concentrations of α-MSH. Intracellular cAMP levels were measured using radioimmunoassay. Results are expressed as the mean ± SEM of triplicate determinations within one experiment. All experiments were performed at least three times (see Table 3 for the number of experiments done for each hMC3R).